Received: November 2017
DOI 10.17677/fn20714807.2018.01.03
Fluorine Notes, 2018, 116, 5-6
Titanium (IV) complexes supported by fluorine-containing [ON] and [ONNO]-types of ligands as catalysts for ethylene polymerization
Vladislav A. Tuskaev a), b), Svetlana Ch. Gagieva a), K.A. Lysenko b),Boris M. Bulychev a)
a Department of Chemistry, M. V. Lomonosov Moscow State University, 1 Leninskie Gory, 119992 Moscow, Russian Federation
b A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 ul. Vavilova, 119991 Moscow, Russian Federation
Abstract: A series of bi- and tetradentate chiral ligands - derivatives of diaryl prolinol, including compounds containing perfluorophenyl rings have been obtained. Novel titanium (IV) complexes were synthesized in high yields from deprotonated by butyllithium ligands and TiCl4. When activated by methylaluminoxane (MAO), complexes exhibited moderate activities (up to 370 kg / mol Ti h atm) toward ethylene polymerization. The introduction of perfluorophenyl moieties, in addition to increasing the activity, leads to an increase in the molecular weight of polyethylene up to the formation of an ultrahigh-molecular weight polymer (2.7-4.2 Da) with a melting point of 142-145 °C.
Keywords: Titanium(IV), fluorinated ligands, Ziegler-Natta polymerization, Ultra-high molecular weight polyethylene
1. Introduction
At present, there is a noticeable increase in interest to a new generation of olefin polymerization catalysts based on transition metals coordination compounds (non-metallocene catalysis [1-7]). Among them, Group 4 non-metallocene catalysts with [NO] and [ONNO]-type phenolic ligands, such as phenoxyimines [8], bis(iminopyrroles) [9], as well as complexes with salan [10], salophane [11] and aminophenol ligands [12] were investigated to the full possible extent. At the same time, the complexes of these metals stabilized by ligands with amino-groups and alcoholic hydroxy groups are much less studied. A few examples of such structures are shown in Figure 1.
We describe here the coordination of [NO] and [ONNO]-type [(2S)-1-[2-[(2S)-2-[hydroxy(diaryl)methyl]pyrrolidin-1-yl]ethyl]pyrrolidin-2-yl]-diphenyl-methanolato ligands to titanium (IV) center as well as the catalytic properties of these compounds in a homogeneous ethylene polymerization reaction.
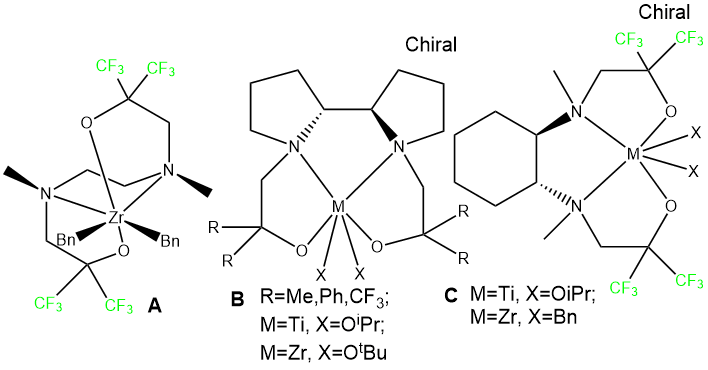
Fig. 1. Examples of amino-alcohol complexes active in olefin polymerization.
2. Results and discussion
We have previously shown that titanium complexes stabilized by tetraaryldioxolan-dimethanol (TADDOL) and 2-hydroxymethylphenol derivatives are effective catalysts for the production of ultra-high molecular weight polyethylene (UHMWPE) [15-17]. To clarify the effect of additional donor nitrogen atoms in ligands on the catalytic activity of coordination compounds, we have synthesized bis-diarylprolinol derivatives. The new ligand systems are bi- [ON] or tetradentate [ONNO]; and aryl substituents are able to create an optimal steric load around the metal center (Scheme 1). The additional donor nitrogen atoms can further stabilize the complexes, thereby hampering the formation of a set of catalytically active sites, leading to the production of polymers with a broad molecular weight distribution.
All these compounds, being derivatives of natural L-proline 1, are chiral, therefore, they can find application not only in the polymerization of olefins, but also in asymmetric catalysis.
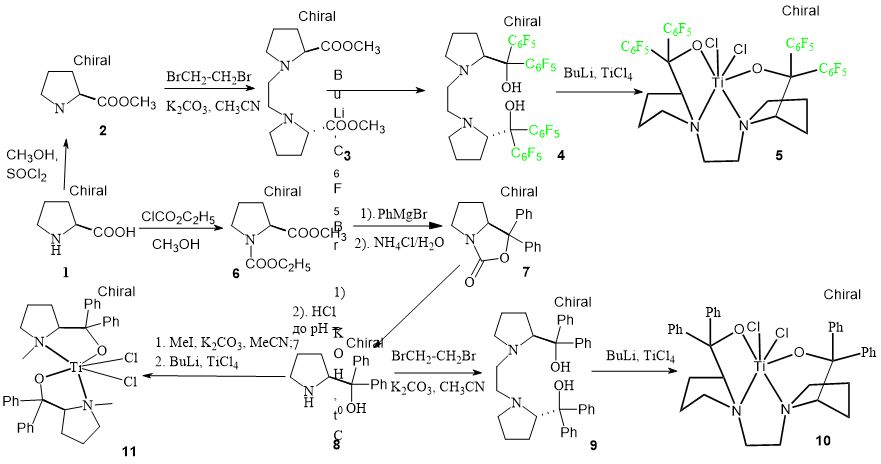
Scheme 1
According to the literature, non-metallocenes contain perfluoroaromatic moieties in the ligand are the most active in the polymerization reaction [6, 8, 13-14]. The tetradentate [(2S)-1-[2-[(2S)-2-[hydroxy(diphenyl)methyl]pyrrolidin-1-yl]ethyl]pyrrolidin-2-yl]-diphenyl-methanol (4) has been synthesized in moderate yield by the reaction of 1,2-bis-[2(S)-2-carbomethoxy-1-pyrrolidinyl]-ethane 3 [18] with perfluorophenyllithium. Its structure has been confirmed by NMR, IR, mass spectroscopy and X-ray diffraction (Fig. 2).
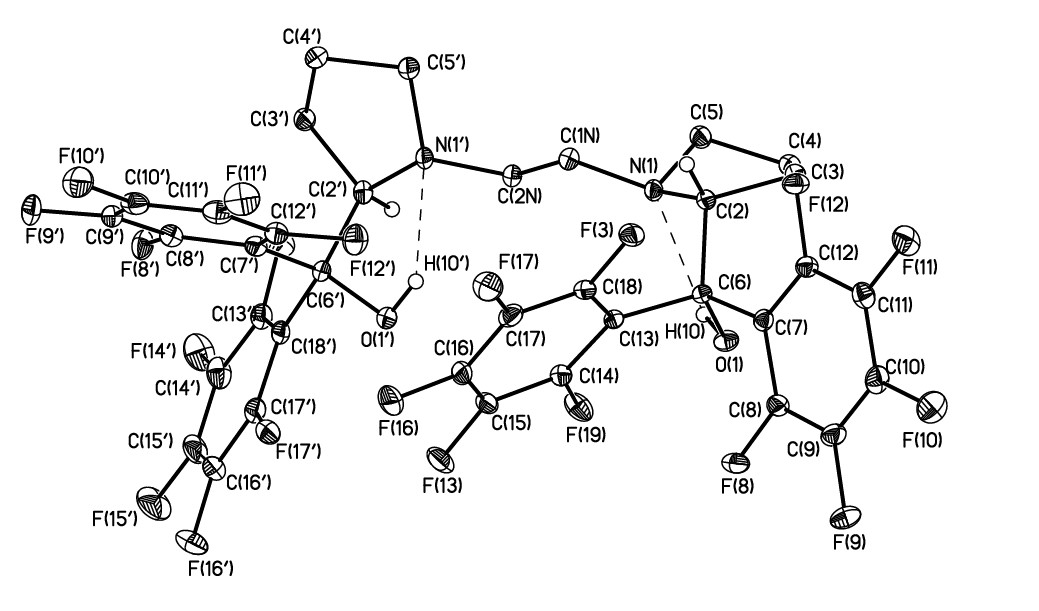
Fig. 2. The general view of 4 in representation of atoms by thermal ellipsoids (p=50%) level. Selected bond lengths (A°) and angles (deg): O(1)-C(6) 1.417(2), N(1')-C(2N) 1.470(2), N(1)-C(1N) 1.467(2), N(1')-C(5') 1.470(2), N(1)-C(5) 1.478(2), N(1')-C(2') 1.485(2), N(1)-C(2) 1.482(2), O(1')-C(6') 1.420(2); C(6)-O(1)-H(1O) 102.9(16), C(2N)-N(1')-C(5') 112.29(14), C(1N)-N(1)-C(5) 112.40(15), C(2N)-N(1')-C(2') 114.50(14), C(1N)-N(1)-C(2) 114.90(14), C(5')-N(1')-C(2') 108.85(14), C(5)-N(1)-C(2) 107.08(14), C(6')-O(1')-H(1O') 104.4(17).
Despite the apparent steric hindrance in 4, fluorinated phenyl substituents and hydroxy groups are located on one side of the median plane of the pyrrolidine rings.
The nitrogen atoms are anti-periplanar with the torsion angle N(1')-C(2N)-C(1N)-N (1) equal to 170.1. The conformation of the pyrrolidine rings is an envelope with the deviation of C(5) and C(4') atoms by 0.56 and 0.60Å. The nitrogen atoms N(1) and N(1') are characterized by a pyramidal environment with a sum of bond angles equal to 334.4 (1) and 335.7 (1)°.
Both hydroxy groups in the molecule 4 participate in the intramolecular H-bonds with the formation of a 5-membered hydrogen bonded cycle The geometric parameters of the H-bonds are somewhat different. In particular, the distance between the donor and proton acceptor N(1)...O(1) and N(1')... O(1') is 2.721 (1) and 2.650 (1) Å, respectively. The observed differences in the strength of H-bonds apparently lead to the above variation of the geometric parameters of the pyrrolidine rings. The shortening of the H-bond O(1')-H(1O')...N (1') is apparently due to intramolecular factors, since the perfluorinated cycles actually completely shield the H-bonded cycle. The most probable reasons may be the presence of a shortened O(1')... C(15) contact (3.165(1) Å) that is characterized by the specific orientation of interacting atoms ((O(1')C(15)C(14) 89.5(1)) and thus can be interpreted as O... .. interaction.
Tetradentate ligand 9 has been synthesized by alkylating of diphenylprolinol 8 (prepared according to a known method [19],) with dibromoethane [20] (Scheme 1). Its structure has been confirmed by NMR, IR, mass spectroscopy.
To check the effect of the C2 linker binding the pyrrolidine rings in the ligands 4 and 9 on the catalytic activity, N-methyldiphenylprolinol was obtained [21] and used as a ligand for the synthesis of the titanium dichloride complex 11.
All the titanium dichloride complexes (5, 10 and 11) have been synthesized by the interaction of lithium derivatives of the corresponding ligands and titanium tetrachloride (Scheme 1) and used in the polymerization of ethylene in-situ, i.e. without their isolation in an individual state (Table 1).
Table 1. Ethylene polymerization by 5, 10 - 11/МАО a
|
Run |
Complex |
[Al]/ [Ti] |
m(pol), g |
A b |
Tm c |
Degree of crystallinity d % |
Mw, e 106 Da |
| 1 |
5 |
500/1 |
0.52 |
347 |
145 |
71 |
4.21 |
| 2 |
5 |
1000/1 |
0.56 |
370 |
142 |
59 |
2.69 |
| 3 |
10 |
500/1 |
0.17 |
113 |
143 |
62 |
2.98 |
| 4 |
10 |
1000/1 |
0.19 |
127 |
142 |
58 |
2.45 |
| 5 |
11 |
500/1 |
0.08 |
53 |
144 |
62 |
3.05 |
a Polymerization conditions: ethylene pressure = 1 atm.; toluene = 10 ml; С(Ti) =3 10-6 mol; incubation time 30 min; temperature 25°C; b Activity, kg of PE mol Ti-1 atm-1 h-1; c Melting temperatures determined by DSC at second heating of UHMWPE samples; d Degree of crystallinity of UHMWPE samples was calculated by use of value Hm100% = 288 J/g [22]; Viscosity-average molecular weight of synthesized UHMWPE samples was calculated with the Mark-Houwink equation: MW = 5.37·104 [η]1.37 [23].
As can be seen from Table 1, the fluorine-containing complex 5 exhibited the highest catalytic activity (up to 370 kg PE mol Ti-1 atm-1 h-1). Its non-fluorinated analogue 10 is three times less active (120 kg PE mol Ti-1 atm-1 h-1). The titanium dichloride complex 11 with two ON ligands exhibited the lowest catalytic activity, 53 kg PE mol Ti-1 atm-1 h-1. Hence it can be concluded that the electronic and geometric parameters of the ligands have a significant effect on the catalytic properties of the coordination compounds.
All catalytic systems based on complexes 5, 10 and 11, are characterized by stable polymerization kinetics. For example, Figure 3 shows the polymerization kinetics on a system with complex 10.
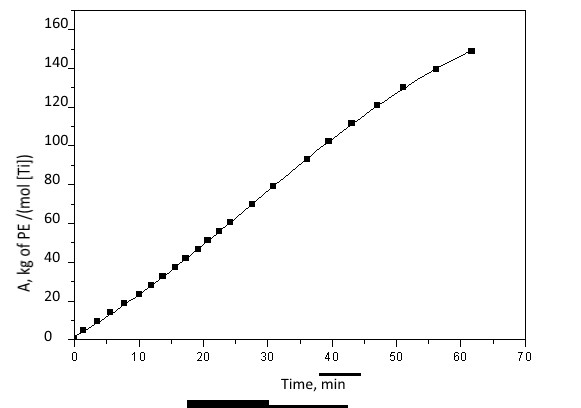
Fig. 3. Kinetics of ethylene polymerization initiated by complex 10 ([Ti]: [MAO] = 1: 500).
3. Conclusion
Thus, the analysis of the presented experimental data allowed us to reveal the following patterns of the relationship between the structure of the complex and its catalytic activity:
- the catalytic activity of Ti (IV) complexes with [ONNO]-type ligands exceeds the activity of the complex with the [ON]-type ligand by more than 2 times;
- the introduction of perfluorophenyl substituents increases the catalytic activity of 3-fold compared to the activity of the non-fluorinated analog.
4. Experimental section
4.1. Materials
All manipulations with air-sensitive materials were performed with rigorous exclusion of oxygen and moisture in oven-dried Schlenk glassware on a dual manifold Schlenk line, interfaced to a high-vacuum line. Argon and ethylene of special-purity grade (Linde gas) were dried by purging through a Super Clean™ Gas Filters. Toluene, hexane THF and diethyl ester were distilled from Na/benzophenone prior to use. The water content was periodically controlled by Karl-Fischer coulometry by using Methrom 756 KF. Unless otherwise noted, all reagents were purchased from Sigma-Aldrich. Methylaluminoxane (MMAO-12, Aldrich) was used as 7 wt% solution in toluene. Compounds 2-3 [18], 6-8 [19], 9 [20] were obtained by known methods.
NMR spectra were recorded on Bruker AMX-400. Deuterated solvent (CDCl3) was degassed by freeze–thaw–vacuum cycles and stored over 3 Å molecular sieves. Chemical shifts are reported in ppm vs. SiMe4 and were determined by reference to the residual solvent peaks. All coupling constants are given in Hertz.
IR spectra were recorded on Magna-IR 750 spectrophotometer. Elemental analysis was performed on Carlo Erba-1106 and Carlo Erba-1108 instruments by the microanalytical laboratory at A. N. Nesmeyanov Institute of Organoelement Compounds.
Optical rotation was measured on a Perkin-Elmer 241 Polarimeter in a 5 cm thermostated cell at 25 °C. For all compounds, the solvent and concentration in grams / 100 ml of solvent are listed.
[(2S)-1-[2-[(2S)-2-[hydroxy-bis(perfluorophenyl)methyl]pyrrolidin-1-yl]ethyl]pyrrolidin-2-yl]-bis(perfluorophenyl)methanol (ligand 4)
A solution of C6F5Br (1.12 ml, 4.5 mmol) in 30 mL of diethyl ether was cooled to -78 °C, and a solution of nBuLi (6.15 ml, 5.0 mmol) was added dropwise. The reaction mixture was stirred at -78 °C for 2 h., and then a solution of compound 3 (1 g, 1.0 mmol) in 10 ml of diethyl ether was added dropwise. The mixture was stirred for 2 h., allowed to slowly warm to room temperature and a saturated aqueous solution of ammonium chloride was added. The organic phase was washed with water; the aqueous phase was extracted twice with methyl tert-butyl ether. The organic phase was dried over anhydrous sodium sulfate; the residual solvent was removed by rotary evaporation. The resulting residue was crystallized from hexane, melting point 194-196 °C (dec.). The yield was 0.8 g (89,5 %). Calculated (%) for C36H20F20N2O2: C, 48.45; H, 2.26; N, 3.14. Found (%):C, 48.39; H, 2.24; N 3.07. 1H NMR (400 MHz, CDCl3), δ: 6.57 (s, 2H), 4.50 (s, 2H), 3.11 (m, 2H), 2.46 (m, 2H), 2.40 (m, 4H), 2.26 (m, 4H), 1.54 (s, 2H), 1.41 (m, 4H).
[(2S)-1-[2-[(2S)-2-[hydroxy(diphenyl)methyl]pyrrolidin-1-yl]ethyl]pyrrolidin-2-yl]-diphenyl-methanol, (ligand 9).
In the argon atmosphere, to the suspension of 1.78 g (12.8 mmol) K2CO3 in 17 ml of acetonitrile, diphenyl-[(2S)-pyrrolidin-2-yl]methanol (3 g, 1.85 mmol) and 1.1-dibromoethane (3.05 ml, 3.95 mmol) were added, the mixture was refluxed for 48 h. At the end of the reaction (determined by TLC), the reaction mixture was cooled to room temperature, the precipitate was filtered off, washed with acetonitrile. The solvent from filtrate was removed by rotary evaporation. The dry residue was recrystallized from hexane: methylene chloride. The product was obtained as white crystals, yielding 2.2 g (40%) with melting point of 150°C.
Calculated (%) for C36H40N2O2: C, 81.17; H, 7.57; N 5.26. Found (%):C, 81.13; H, 7.54; N 5,20. 1H NMR (400 MHz, CDCl3), δ: 7.29-7.23 (m, 20 H), 4.5 (s, 2Н), 3.32 (s, 2 Н), 2.40 (m, 4 Н), 2.35 (m, 4Н), 1.65 (m, 4 Н).13C NMR (125 MHz,) δ 146.12, 128.26, 127.78, 127.75, 76.76, 75.86, 56.86, 56.63, 28.32, 24.18.
Synthesis of the complexes were carried out according to the following procedure:
A solution of ligand 4, 8а or 9 (0.20 mmol) in 20 mL of toluene was cooled to -78 °C, and 0.168 mL of nBuLi (2.5 M solution in hexane, 0.42 mmol) was added dropwise. The reaction mixture was stirred at -78 °C for 15 min, allowed to slowly warm to room temperature, and then stirred for 2 h. Neat titanium tetrachloride (22 μL, 0.20 mmol) was added dropwise at-30 °C and the mixture was stirred overnight. The solvent was removed in vacuo, and complexes were recrystallized from toluene: hexane 1:1.
X-ray crystal structure determination. X-ray diffraction data for 4 was collected on a Bruker Smart APEX II CCD diffractometer (graphite monochromator, λ(MoKα) = 0.71073 Å, temperature 100 K, -scanning, 2θmax = 58º). Colorless crystals of C36H20F20N2O2 at 100 K are triclinic, space group P-1, Z = 2, а = 11.0812(12), b = 12.1050(18), c = 13.692(2) Å, 69.925(3), β = 81.771(4), = 73.776(3), V = 1654.1(4) Å3, dcalc = 1.792 g cm-3.
Intensities of 8716 independent reflections out of 19736 collected were used in structure solution and refinement.
The structure was solved by direct methods and refined by the full-matrix least-squares technique against F2 in the anisotropic approximation. The hydrogen atoms were placed in the geometrically calculated positions and included in the refinement according to the “riding” model.
The refinement converged to R1 = 0.0452 (calculated for 5841 observed reflections with I>2σ(I)), wR2 = 0.1155 and GOF = 1.018. All calculations were performed with SHELXTL PLUS software package [Sheldrick GM. Acta Cryst 2008;A64:112.].
Atomic coordinates, bond lengths and angles and thermal parameters have been deposited at the Cambridge Crystallographic Data Center (CCDC), reference 1587715. Copies of the data can be obtained can be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44(0)1223-336033.
Atomic coordinates, bond lengths and angles and thermal parameters have been deposited at the Cambridge Crystallographic Data Center (CCDC), reference 1587715. Copies of the data can be obtained can be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44(0)1223-336033.
Polymerization of ethylene was carried out under atmospheric pressure in a jacketed 100 mL stainless steel reactor equipped with a mechanical stirrer, a temperature controller and inlets for loading components of catalytic systems and ethylene. The reactor was kept under vacuum for 1 h at 50 °C before each experiment. Toluene and the necessary amount of cocatalysts were loaded into the nitrogen-purged reactor and stirred vigorously. The reactor was heated to a specified temperature and reaction mixture was saturated with ethylene to achieve a total ethylene and solvent vapors pressure of 1 atm. Polymerization was initiated by addition of precatalyst to the reaction mixture. The pressure of ethylene was maintained constant during polymerization; temperature was thermostatically controlled as indicated. After a desired period of time, the reactor was vented. Polymerization was stopped by addition of 10% HCl solution in ethanol to the reactor. The polymer was filtered out, washed several times with water-ethanol mixture, then immersed in acidified ethanol for 12 h to remove inorganic impurities, and then dried under vacuum at 50–60 °C until a constant weight was achieved.
Viscosity-average molecular weight of synthesized UHMWPE samples was calculated with the Mark-Houwink equation: MW = 5.37·104 [η]1.37 [23], where: MW = viscosity-average molecular weight (g/mol); [η] = intrinsic viscosity in decalin at 135°C (dl/g); [η]= (2ηsp -2lnηr )1/2/0.056 (ηsp - specific viscosity decalin at 135°C; ηr - relative viscosity in decalin at 135°C; ηr = ηsp +1.
Acknowledgments
This work was financially supported by the Russian Science Foundation (Project No 16-13-10502).
References
- Park S., Han Y., Kim S. K., Lee J., Kim H. K., Do Y., J. Organomet. Chem. 2004, 689, 4263–4276.
- Busico V., Dalton Trans., 2009, 41, 8794–8802.
- Takeuchi D., Dalton Trans., 2010, 39, 311–328.
- Budagumpi S., Kim K.-H., Kim I. Coord. Chem. Rev., 2011, 255 2785– 2809.
- Delferro M. and Marks T. J., Chem. Rev. 2011, 111, 2450–2485.
- Bryliakov K. P., Talsi E. P., Coord. Chem. Rev. 2012, 256, 2994– 3007.
- Baier M. C., Zuideveld M. A., and Mecking S., Angew. Chem. Int. Ed. 2014, 53, 9722 – 9744.
- Makio H., Terao H., Iwashita A., and Fujita T., Chem. Rev., 2011, 111, 2363–2449.
- Mashima K., Tsurugi H., J. Organomet. Chem. 2005, 690, 4414–4423.
- Cohen A., Kopilov J., Lamberti M., Venditto V. and Kol M., Macromolecules, 2010, 43, 1689–1691.
- Gendler S., Zelikoff A. L., Kopilov J., Goldberg I. and Kol M., J. Am. Chem. Soc., 2008, 130, 2144–2145.
- Groysman S., Sergeeva E., Goldberg I., and Kol M., Inorg. Chem., 2005, 44, 8188–8190.
- Kirillov E., Lavanant L., Thomas C., Roisnel T., Chi Y., and Carpentier J.-F., Chem. Eur. J. 2007, 13, 923 – 935.
- Sergeeva E., Kopilov J., Goldberg I. and Kol M., Inorg. Chem., 2009, 48, 8075–8077.
- Tuskaev V. A., Gagieva S. Ch., Maleev V. I., Borissova A. O., Solov’ev M. V., Starikova Z. A., Bulychev B. M., Polymer 2013, 54, 4455-4462.
- Gagieva S. Ch, Tuskaev V. A., Fedyanin I. V., Buzin M. I., Vasil'ev V. G., Nikiforova G. G., Afanas'ev E. S., Zubkevich S. V., Kurmaev D. A., Kolosov N. A., Mikhaylik E. S., Golubev E. K., Sizov A. I., Bulychev B. M., J. Organomet Chem 2017, 828, 89-95, http://dx.doi.org/10.1016/j.jorganchem.2016.11.026.
- Gagieva S. Ch., Tuskaev V. A., Fedyanin I. V., Sizov A. I., Mikhaylik E. S., Golubev E. K., Bulychev B M., Polyhedron 2017, 122, 179–183, http://dx.doi.org/10.1016/j.poly.2016.11.007.
- Yang X., Li B. and Fu E. Synthetic Communications, 35, (2005) 271–278, DOI: 10.1081/SCC-200048458.
- Mathre D. J., Jones T. K., Xavier L. C., Blacklock T. J., Reamer R. A., Mohan J. J., Jones E. T. T., Hoogsteen K., Baum M. W., and Grabowski E. J. J., J. Org. Chem., 1991, 56, 751-762.
- Xu Q., Zhu G., Pan X., Chan Han A. S.C. Chirality, 2002, 14, 716–723.
- Braga A. L., Paixao M. W., Westermann B., Schneiderc P. H. and Wessjohann L. A. J. Org. Chem. 73, (2008), 2879–2882, DOI: 10.1021/jo702413n
- Wunderlich B., Cormier C.M., J. Polym. Sci., Part A-2: Polym. Phys. 5 (1967) 987-988.
- The UHMWPE handbook: ultra-high molecular weight polyethylene in total joint replacement / Steven M. Kurtz., 2004, Elsevier Academic Press.
- Sheldrick GM. Acta Cryst 2008;A64:112.
Recommended for publication by Prof. S. M. Igumnov
Fluorine Notes, 2018, 116, 5-6
