Received: March 2018
DOI 10.17677/fn20714807.2018.02.02
Fluorine Notes, 2018, 117, 3-4
Application of Alkaline Metal Fluorides for Doping of Aluminum
Skachkov V.М., Pasechnik L.А., Yatsenko S.P.
Institute of Solid State Chemistry UB RAS,91, Pervomaiaskaya st, Ekaterinburg, 620990 (Russia)
Abstract. High-temperature exchange reactions between liquid aluminum and fluoride salt
systems containing scandium, yttrium, zirconium, and hafnium compounds have been studied. High-temperature
reactions in other salt systems have been compared. Advantages and disadvantages of application of
alkaline metal fluorides for doping of aluminum have been shown. Keywords: salt melt, aluminum, scandium, yttrium, zirconium, hafnium, high-temperature
exchange reactions In construction, mechanical engineering, aircraft building, rocket production, and many other sectors
of the national economy they use mainly aluminum alloys since pure aluminum has poor technical characteristics,
and doping of aluminum alloys with rare-earth scattered elements finds an ever-greater application,
which considerably improves their service properties [1,2]. Small additions of scandium, yttrium,
zirconium, and hafnium restrict grain growth of aluminum alloys, stabilize the crystal structure
at high temperatures, increase the mechanical and corrosion properties, and improve weldability and
plasticity. The presence of only 0.3 wt.% Sc increases the tensile strength of annealed aluminum
sheets from 55 to 240 MPa, and in Al-5%Mg alloy – from 260 to 400 MPa. The effect of scandium manifests
itself at smaller concentrations in the presence of zirconium. Yttrium additions of not more than
0.1 wt.% reduce the oxidation rate, especially in hard aluminum alloys. Hafnium in aluminum alloys
binds such harmful impurities as iron, alkaline metals etc. into intermetallic compounds (IMC). The
introduction of 1 wt.% Hf leads to the production of ultrastrong alloys exhibiting enhanced resistance
to vibrational damage; such alloys have a grain size of ~ 40-50 nm [3-10]. Aluminum is usually doped
by introducing master alloys, that can be obtained by different methods, into a melt. A good review
of scandium doping methods is given by researchers of the Institute of High-Temperature Electrochemistry
UB RAS in work [11]. The aluminothermal method for doping of aluminum
and aluminum-based alloys, both castable and wrought, holds much promise. It is not obvious that
high-temperature exchange reactions in salt melts can be used to introduce refractory, scattered,
and rare metals into aluminum. The authors have doped aluminum with small additions (less than
1 wt.%) of scandium, yttrium, zirconium, and hafnium. Melting of salts and high-temperature exchange reactions were carried out in a Nabertherm L 9/11 muffle
furnace. X-ray phase
analysis (XPA) of melted (reaction) salts was performed on Shimadzu and DRON-2.0 diffractometers
in CuK radiation with the angle interval 10° ≤ 2Θ ≤ 70°, a scanning step of 0.03°, and 2 s time in point.
The phases were identified using the Powder Diffraction File JCPDSD-ICDD PDF2 (sets 1-47). The produced
reaction salts were ground in an agate mortar and stored in an desiccator. The high-temperature exchange
reactions were carried out in alundum crucibles at 750-780 °С; the salts were fed into the aluminum
melt by means of a laboratory-scale injection plant (Fig. 1), and bottled carbon dioxide served as
a transporting agent. The elemental analysis of the melts was performed on a Spectromass 2000 mass-spectrometer
with inductively bound plasma. Granular aluminum (p.a.) TU 6-09-02-529-92;
NaF (p.a.) GOST 4463-76; KF (pure) GOST 20 848-75; Sc2O3 (pure) TU 48-4-417-87;
Y2O3 (pure) TU 48-4-191-72; ZrO2 (pure) TU 6-09-2486-77; HfO2 (pure) TU 48-4-201-72 were used for synthesis. The application of aluminothermal methods for the production of master alloys and alloys by injection
of a gas-powder suspension through aluminum melt has been comprehensively examined by the authors
and described in [12]. The suspension consists of inert gas (argon Ar or carbon dioxide CO2)
and a ground salt mixture of sodium fluoride and potassium chloride with additions of aluminum fluoride,
which contains oxides or fluorides of metals introduced into the alloy at 700-900 С. A corresponding
patent [13] has been acquired, and aluminum-scandium alloys have been obtained by the injection method
in industrial furnaces at JS KUMZ, which was described in [14]. Mixtures of alkaline metal salts dissolve oxides and fluorides of other metals during melting forming
complex compounds that easily interact with molten aluminum. This makes it possible not to use
such expensive compounds as for example potassium fluozirconates (hafnates). The application
of sodium and potassium fluoride melts for the dissolution of oxides holds much promise, which
is demonstrated with an example of scandium oxide solubility in different salt systems (Fig. 2,
curve 8). It was determined from the XPA data (Fig. 3) that scandium oxide dissolves
during melting with sodium and potassium fluorides forming 2-potassium sodium hexafluoroscandiate
(scandium cryolite-elpasolite) K2NaScF6. Yttrium oxide behaves in similar
salt mixtures like scandium oxide. Zirconium and hafnium dissolve giving rise to oxyfluorides
of variable composition, in much the same way as they behave during the formation of oxyfluorides
from their dioxides when treated with concentrated solutions of hydrofluoric acid. As a result,
Hf(Zr)F4∙nH2O is precipitated, and oxyfluorides are formed during drying
in air at 200-250 С. The products of their thermal decomposition are Hf4F12O2 and Zr4F10O3. A large number of salt systems (Fig. 2) based on fluoride-chloride salts of alkaline (Li, Na,
K) and alkaline-earth (Сa) metals with introduced scandium, yttrium, zirconium, and hafnium fluorides
or oxides have been studied to search for the optimal conditions of their displacement with more
active aluminum. The eutectics melting temperatures vary depending on the composition of salt
mixtures from 454 °С (for LiF-NaF-KF) to 965 °C (cryolite Na3AlF6)
[15]. The use of calcium salts allows a considerable reduction of the concentration of sodium
in the final alloy, whose admixture may substantially increase the volume of rejects in the production
of rolled stock. We have established the solubility values of scandium fluoride and oxide as
a function of temperature; the application of scandium oxide lowers slightly its direct yield
in the solution compared with scandium fluoride. For example, at 800 °С the solubility value
in a calcium salt mixture is 0.6 wt.% for Sc2O3 and 3.4 wt.%
for ScF3. The application of sodium and potassium fluorides instead of chlorides not only increases the reactivity
in high-temperature exchange reactions, but also reduces metal loss during evaporation since
fluorides are less volatile than chlorides, although fluorides have higher melting temperatures
(Table 1) [16,17]. Table 1. Melting and boiling temperatures of metal chlorides and fluorides Substance Melting temperature t,°C Boiling temperature t,°C LiCl LiF 614 870 1380 1681 NaCl NaF 801 992 1465 1705 KCl KF 776 857 1406 1500 CaCl2 CaF2 772 1418 1600 2500 AlCl3 AlF3 (192,6) under pressure (1290) under pressure sublimates 181,2 sublimates 1272 ScCl3 ScF3 956 1552 975 1607 YCl3 YF3 703 1155 1510 - ZrCl4 ZrF4 (437) under pressure (910) under pressure sublimates 333 sublimates 903 HfCl4 HfF4 (432) under pressure - sublimates 315 sublimates 974 The eutectic 0.4NaF + 0.6KF melts at 721 °С [18], which allows the exchange reactions
to be carried out at temperatures below 800 °С. The thermodynamic calculations for the
displacement reaction, for example, displacement of scandium by aluminum into alloy at 800 °С,
give a value of yield of ~80%. It was determined experimentally that an increase in the temperature
of interaction lowers the yield of scandium from the salt melt, whereas the process temperature
of 700-750 °С provides a direct yield of scandium into alloy of 95% and more. The economic effectiveness of production of alloys with unique properties (mechanical, thermal, corrosion,
radiation etc.) can be enhanced by injection of prepared reaction powder into liquid aluminum.
Vigorous agitation of alloy during injection promotes uniform distribution of introduced components
and acceleration of the high-temperature reaction, which considerably reduces the doping time
in whole. Besides, some impurities are removed from aluminum. The chemical composition of the
alloy impurities during doping of A85 grade aluminum was determined as (initial Al – numerator,
after melting – denominator, wt.%): Cu - 0.01/0.0019; Mg - 0.01/0.0007; Zn - 0.01/0.001; Mn -
0.01/0.003; Si - 0.05/0.038; Na - 0.0014/0.0012; and hydrogen < 0.29 m3/100 g. On completing the injection with carbon dioxide and 5 min exposure, the slag was discharged (730-750 °С)
and the obtained alloy was poured out into a cast-iron mold lined with hexagonal boron nitride.
An averaged alloy sample was taken and analyzed; the results are presented in Table 2. Table 2. Interaction of liquid aluminum with scandium, yttrium, zirconium, and hafnium
oxides/fluorides/oxyfluorides dissolved in fluoride melt (0.4NaF + 0.6KF) No. Oxide, fluorides In alloy, % Direct yield, % Sc Y Zr Hf Sc Y Zr Hf 1 Sc2O3 0.91 - - - 93 - - - 2 ScF3 0.98 - - - 96 - - - 3 Y2O3 - 0.95 - - - 61 - - 4 YF3 - 1.15 - - - 69 - - 5 ZrO2 - - 1,00 - - - 92 - 6 Zr4F10O3 - - 1,05 - - - 94 - 7 HfO2 - - - 0,9 - - - 82 8 Hf4F12O2 - - - 1,1 - - - 88 The mass ratio of the fluoride melt to the aluminum melt during melting was determined by the
solubility of the chosen metal oxides. For the salt/metal ratios less than 0.5 for Zr, 0.8
for Sc, and 1.0 for Y and Hf, the process of reduction of rare metals is inhibited, therefore
the components pass into the alloy to a lesser extent, while salt/metal ratios of more than
1.2 lead to efficiency loss and a larger volume of recycling salts. Replacement of oxides
to fluorides and oxyfluorides of the metals of the input increases direct access from 2 to
7%. The injection method of alloy production allows one to combine necessary amounts of different
ingredients in the form of fluoride and oxide compounds in the injected powder. Metals, that
are electrochemically more positive than aluminum, can be reduced simultaneously, for example,
scandium and zirconium, scandium and hafnium, yttrium and zirconium, yttrium and hafnium
etc., which further facilitates the technology of alloy production. Note that the reaction
powder should be produced by preliminary alloying of fluorides with the corresponding oxides
or fluorides and subsequent grinding. Ground powder is very hygroscopic and should be used
immediately or stored in a damp-proof place. Recommended for publication by Prof. S.M. Igumnovе-mail: vms@weburg.me
Introduction
Experimental
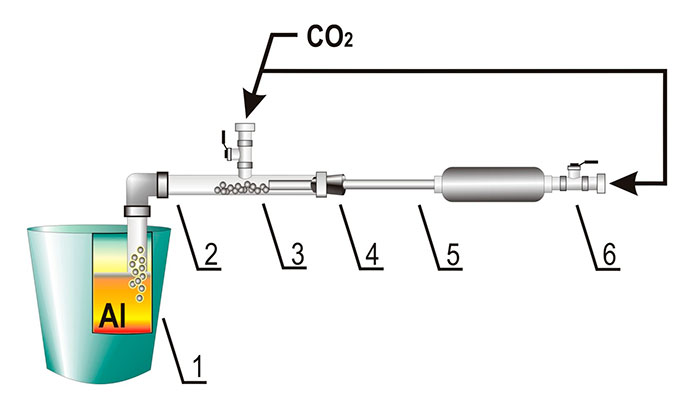
Fig. 1. The diagram of the laboratory plant for injection of reaction salts into aluminum salt: 1 – crucible for aluminum; 2 – single-nozzle tuyere; 3 – salt powder; 4 – plug; 5 – accelerating nozzle;
6 – pulse gate; СО2 – protective gas; Al – liquid aluminum
Results and discussion
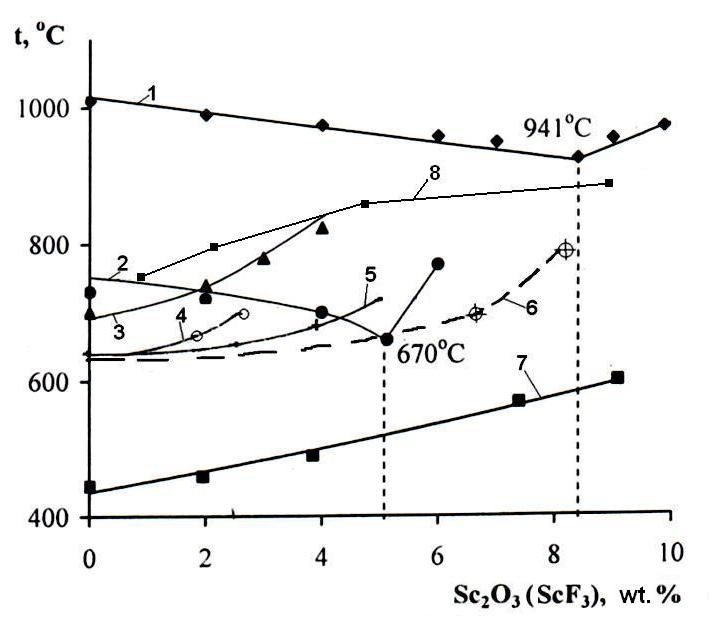
Fig. 2. Segments of liquidus curves of salt systems with scandium oxide and fluoride: 1 - Na3AlF6 – Sc2O3; 2 - (0.53NaF + 0.47AlF3)
– Sc2O3; 3 - (0.09Na5Al3F14 +
0.91KCl) – Sc2O3; 4 - (0.86CaCl2 + 0.14CaF2)
– Sc2O3; 5 - (0.86CaCl2 + 0.14CaF2) – ScF3;
6 - (0.82Li3AlF6 + 0.18K3AlF6) – ScF3;
7 - (0.59KF + 0.29LiF + 0.12NaF) – ScF3; 8 – (0.4NaF + 0.6KF) – Sc2O3
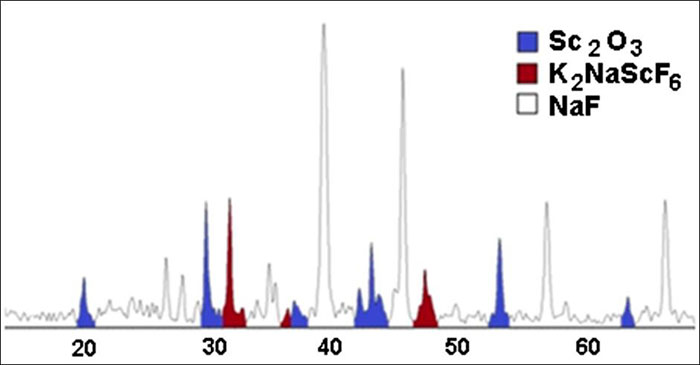
Fig. 3. XRD pattern of salt system with scandium oxide: (0.4NaF + 0.6KF) – Sc2O3
Note: The presented results are average data of three identical meltings.
The work was carried out in accordance with the state assignment and R&D plans of the ISSC
UB RAS (No. AAAA-A16-116122810213-2).
References
Fluorine Notes, 2018, 117, 3-4
