Received: April 2018
DOI 10.17677/fn20714807.2018.03.02
Fluorine Notes, 2018, 118, 3-4
Interaction of allyl esters of alcohols-telomeres (n = 1, 2, 3) with maleopimaric acid
L.M. Popova1, A.S. Tsyrulnikova1, S.V. Vershilov2, I.U. Butko1
1Saint Petersburg State University of Industrial Technologies and Design, Higher School of
Technology and Energetics
Ivan Chernykh Street, 4, St. Petersburg, 198095 (Russia)
е-mail: lorapopova@mail.ru
2Federal State Unitary Enterprise Scientific And Research Institute of Synthetic Rubber named
after Academic S. V. Lebedev
Gapsalskaya street, 1, St. Petersburg, 198035 (Russia)
Abstract:The synthesis of esters was carried out by the reaction of maleopimaric acid with 6,6,7,7-tetrafluoro-4-oxahept-1-ene, 6,6,7,7,8,8,9,9-octafluoro-4-oxanon-1- ene and 6,6,7,7,8,8,9,9-dodecafluoro-4-oxaundec-1-ene under conditions of acid catalysis.
Keywords: alkylation, allyl ethers of telomeres (n = 1, 2, 3), maleopimaric acid.
Rosin and the products of its chemical transformations such as derivatives of maleopimaric acid are actively studied and used in various fields for decades [1-4]. Thus, rosin-based compounds are used as adhesives in the paint and varnish industry for the production of artificial rubbers in electrical engineering and other fields.
It is known that the introduction of fluorine atoms into an organic molecule significantly affects the physicomechanical properties of substances: it increases chemical and heat resistance, resistance to light, and mechanical treatments. The introduction of fluorine atoms affects the electrical conductivity, foaming and emulsification ability.
This research is derived to acylation of allyl ethers of alcohol-telomeres investigation when resin acids derivatives applied [8].
The interaction of maleopimaric acid with (polyfluoroalkoxy) allyl ethers (a-b) was carried out under acid catalysis for 20 h without any solvent (IIa-c) and in presence of toluene as a solvent (IIb) at heating (104-158 °C) (Sch.1). The reaction was monitored by TLC. At the end of the process, the stock was dissolved in diethyl ether and washed with distilled water until neutral pH and was dried with anhydrous sodium sulfate. The crystalline substances (IIa-b) of a light brown color (yields 40-89%) were obtained after removal of the solvent and dried under vacuum over P2O5.
Scheme 1
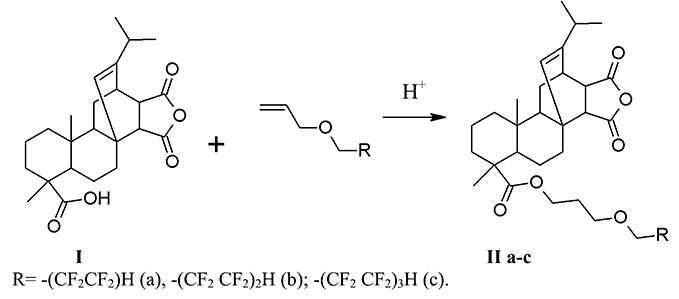
The NMR 1H spectra of products (II a-c) contain proton signals of -CF2H- group in the form of triplet of triplets with a chemical shift 5.96 ppm (for II a), 6.08 (for II b), and 6.09 ppm (3JH-F=52 Hz , 2JH-F=4 Hz) (for II c). The proton signals in the form of triplet of ester groups –С1Н2О at 3.83 (for II a) at 3.94 ppm (for II c), 3.95 ppm (for II b) indicate the undergoing of the reactions. The protons’ resonance signals of other (polyfluoroalkoxy) allyl fragments are also present. They correspond to the structure of the ether by their chemical shifts, multiplicity and integral intensities. The analysis of the NMR 1H spectra indicates that the reaction also proceeds against the Markovnikov’s rule [8].
In the NMR 19F spectra of products (II a-c), the following resonant signals of fluorine atoms of difluoromethylene groups of the alkyl fragment are observed: for the product (II a) at – 125.40 ppm and – 140.01 ppm. in the form of doublet (J=51 Hz); for the product (II b) ̶ C6F2, C7F2, C8F2 in the form of singlets with chemical shifts, respectively, 119.54, – 125.17 and – 129.85 ppm, and C9F2 as doublets at – 133.14 ppm. (J=50.8 Hz), for the product (II c): C6F2 and C7F2 – singlets with chemical shifts - 119.35. and 122.16 ppm, C8F2 and C9F2 - doublet with δF = 123.35 ppm (J=46.9 Hz), C10F2 ̶ singlet with δF = 129.35 ppm and C11F2H as a doublet with δF – 137.08 ppm. (J=46.98 Hz). The signals correspond to the ascribed structures by the chemical shifts and integrated intensities.
There are bands of stretching vibrations of С-Н bonds in the range of 3028-2872 cm-1, bands of stretching vibrations of carbonyl group С=О at 1778 cm -1, stretching vibrations of double bond C=C at 1693 cm-1, stretching vibrations of ether C-O-C bond in the range of 1250-1249 cm-1 and stretching vibrations of C-F in the interval 1130-1084 cm-1 in the IR spectrum of products (II).
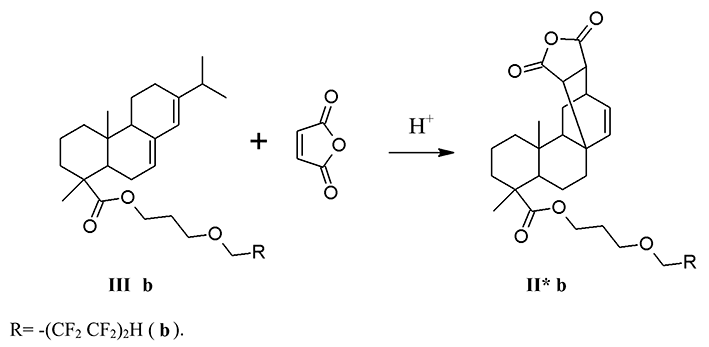
The counter synthesis of the product (II* b) was carried out by the reaction of diene synthesis of 3-(1,1,2,2,3,3,4,4-octafluoropentoxy) propylabietate (III b) with maleic anhydride without any solvent at a temperature of 147-152 °C for 2 h (sch. 2).
Scheme 2
The reaction was monitored by TLC. At the end of the process, the stock was dissolved in diethyl ether,
washed with distilled water and dried with anhydrous sodium sulfate. The crystalline product of a
dark brown color (II *b) was obtained (raw yield 89%) after removal of the solvent and drying under
vacuum over P2O5.
In the NMR 1H spectrum of the products (II* b) there are multiplet signals of the polyfluoroalkyl fragment in the form of triplet triplets with chemical shifts at 6.09 ppm corresponding to the protons of CF2H group. The proton signals of ester groups -CH2O- are observed at 3.94 ppm. The formation of the diene synthesis product is indicated by the signal with a chemical shift at 5.56 ppm.
In NMR 19F spectrum of the product (II* b), there are resonant signals corresponding to the polyfluoroalkyl fragment by the chemical shifts, multiplicities and integrated intensities in the range of 137.14 ÷ 119. 54 ppm.
In the UV spectrum of the ether (II * b) the absorption maxima corresponding to the π → π * transition in the 210 nm intervals (lgε 5.26) and 260 nm (lgε 5.18) appear in alcohol solution.
Experimental
The UV spectra of alcohol solutions of compounds were obtained using spectrophotometer SF-2000 at the concentration of 10-4 mol/l, the thickness of the absorbing layer is 1 cm.
The NMR 1H and 19F spectra were registered using Bruker 500 with the operating frequency of 500 MHz for 1H (470 MHz for 19F), in solutions of CDCl3 and DMSO-d6. The internal standard is TMS, the external is CCl3F.
The IR spectra were recorded by Shimadzu IRPrestige-2. The measurements were analyzed on KBr glasses (the solutions in CHCl3 and CCl4).
The process of these reactions and the purity of starting and resulted compounds were carried out by TLC on Sorbfil plates, the eluent: hexane-methylene chloride-acetone (1: 1: 0.5) or hexane-methylene chloride (1:1).
The freshly distilled allyl ethers were used at this research ̶ 6,6,7,7-tetrafluoro-4-oxahept-1-ene (a) (b.p. 110-112°C), 6,6,7,7,8, 8,9,9-octafluoro-4-oxanone-1-ene (b) (b.p. 141°C) and 6,6,7,7,8,8,9,9,10,10,11, 11-dodecafluoro-4-oxaundec-1-ene (c) (b.p. 171.8°C), synthesized according to the method [9], and also maleopimaric acid (I) (mp 218°C), received likewise [10], maleic anhydride (mp 52.8°C) and 3- (1,1,2,2,3,3,4,4-octafluoropentoxy) propylabietate (IIIb) synthesized by the method [8].
6,6,7,7-Tetrafluoro-4-oxaheptyl ester of maleopimaric acid (IIa). 0.8 g (6.0 mmol) of 6,6,7,7-tetrafluoro-4-oxahept-1-ene (a) and 2 drops of concentrated H2SO4 were added to 2.0 g (5.0 mmol) of maleopimaric acid (I), and was heated at 104-110 °C for 20 h. At the end of the process, the stock was cooled, dissolved in diethyl ether, washed with distilled water until neutral pH. The organic layer was separated and dried with Na2SO4. The solvent was distilled off and the residue was dried in vacuum over P2O5. Yield: (IIa): 2.37 g (89%), light brown crystalline solid, mp 94-102 °C, Rf 0.45 (C6H12-CH2Cl2-CH3COCH3, 1:1:0.5). IR spectrum (CCl4), Cm-1: 3028–2871(νC-Н).1777 (νC=O, anhydride); 1730 (νC=O, ester.); 1696 (νC=C); 1204 (νC-O-C); 1126-1016 (νCF). UV spectrum (EtOH), nm (lg ε): 208 (4.71), 235 (4.53), 260 (5.73). NMR 1H spectrum, δ, ppm: 1.02 (d, J=6 Hz); 1.26 (dd, J1=19 Hz, J2=8 Hz); 2.20 (s 1H C15H); 2.56 (d J=13 Hz, 2.75 d J=8 Hz;) 3.83 (t 2H, OCH2 ester., J1=12 Hz); 4.11 (t 2H, CH2O, J=6 Hz); 4.12 (t 2H, CH2O, J=6 Hz); 5.57 (s 1H C14H); 5.96 (tt 1H, CF2H, J1=53 and J2=6 Hz). NMR 19F spectrum, δ, ppm: − 140.01 (d 2F, C5F2, J=51Hz) −125.40 (s 2F, CF2).
6,6,7,7,8,8,9,9-Octaftor-4-oxanonyl ester of maleopimaric acid (IIb). The product (IIb) was obtained in a similar manner to the product (IIa) from 3.5 g (8.75 mmol) (I), 2.86 g (10.5 mmol) of 6,6,7,7,8,8,9,9-octafluoro-4-oxanone-1-ene ), 3 drops of concentrated H2SO4, 20 ml of toluene, at 116-118°C for 20 h. Yield: (IIb): 2.37 g (40%), dark brown crystalline solid, mp 136-142 °C, Rf 0.56 (C6H12-CH2Cl2-CH3COCH3, 1:1:0.5). UV spectrum (EtOH), nm (lg ε): 212 (6.42), 247 (shoulder). NMR 1H spectrum, δ, ppm: 0.63 (s 3H C20H3); 1.03 (d 6H, C16H3 J=6 Hz); 1.26 (dd J1=20 Hz, J2=7 Hz); 2.20 (s 1H C15H); 3.95 (t 2H, OCH2 ester, J=14 Hz); 4.11 (t 2H, OCH2, J=6 Hz); 4.16 (t 2H, OCH2, J=6 Hz); 5.56 (s 1H, C14H), 6.09 (tt 1H, CF2H, J1=52 Hz, J2 = 4 Hz). NMR 19F spectrum, δ, ppm: - 137.14 (d 2F, C9F2, J=50.8Hz); - 129.85 (s 2F, C8F2); - 125.17 (s 2F, C7F2); - 119.54 (s 2F, C6F2).
6,6,7,7,8,8,9,9,10,10,11,11-Dodecafluoro-4-oxaundecanyl ester of maleopimaric acid (IIc). The product (IIc) was obtained in a similar manner to the product (IIa) from 2.0 g (5.0 mmol) of (I), 2.06 g (5.5 mmol) of 6,6,7,7,8,8,9,9,10,10,11,11-dodecafluoro-4 -oxaundec-1-ene (c), 3 drops of concentrated H2SO4, at 155-158°C for 20 h. Yield: 1.93 g (50%), crystalline substance of dark brown color, mp 138-143 °C, Rf 0.61 (C6H12-CH2Cl2-CH3COCH3, 1:1:0.5). UV spectrum (EtOH), nm (lg ε): 214 (6.15), 256 (shoulder). NMR 1H spectrum, δ, ppm: 0.89 (s 3H C20H3); 2.20 (s 1H C15H); 3.94 (t 2H, OCH2 ester, J=14 Hz); 4.15 (t 2H, OCH2, J=6 Hz); 4.16 (t 2H, OCH2, J=6 Hz); 5.57 (s 1H C14H); 6.08 (tt 1H, CF2H, J1=52 Hz, J2=4 Hz). NMR 19F spectrum, δ, ppm: - 137.08 (d 2F, C11F2H, J=46.98 Hz); - 129.35 (s 2F, C10F2); - 123.35 (s 4F, C8F2C9F2); - 122.16 (s 2F, C7F2); - 119.35 (s 2F, C6F2).
The counter synthesis of 6,6,7,7,8,8,9,9-octafluoro-4-oxanonyl ester of maleopimaric acid (II*b). 0.51 g (5.2 mmol) of maleic anhydride, 3 drops of concentrated H2SO4 were added to 2.5 g (4.4 mmol) of 3-(1,1,2,2,3,3,4,4-octafluoropentoxy) propylabietate (IIIb) and was heated at 147-152°C for 2 h. The reaction was monitored by TLC. At the end of the process, the resulting product was cooled, dissolved in toluene, washed with distilled water until neutral pH. The organic layer was separated and dried with Na2SO4. Toluene was distilled off, and the residue was dried in vacuum over P2O5. Yield (II*b): 2.65 g (90%), crystalline sand-colored substance, mp 123-127°C, Rf 0.67 (C6H12-CH2Cl2-CH3COCH3, 1:1:0.4). IR spectrum, cm-1: 3027-2651 (νC-Н); 1778 (νC=O, anhydride); 1735 (νC=O, ester); 1695 (νC=C); 1229 (νC-O-C); 1204; 1190-948 (νСF). UV spectrum (EtOH), nm (lg ε): 210 (5.26), 260 (5.18). NMR 1H spectrum, δ, ppm: 0.65 (s, 3H, C20H3); 1.02 (d 6H, C16H3, J=6.88 Hz); 1.19 (s 3H, C19H3); 1.81 ÷ 1.41 (12H, CH2); 2.27 (m 1H, C15H); 2.55 (d 2H, C7H2, J=13.6 Hz); 2.76 (d 1H, C21H, J=8.56 Hz); 3.94 (t 2H, OCH2 ester, J=14 Hz); 4.11 (t 2H, OCH2, J=6 Hz); 4.16 (t 2H, OCH2, J=6 Hz); 5.56 (s 1H, C14H); 6.09 (tt 1H, CF2H, J1 = 52 Hz, J2 = 4 Hz). NMR 19F spectrum, δ, ppm: - 137.14 (d 2F, C9F2 J=50.8 Hz); - 129.85 (s 2F, C8F2); - 125.17 (s 2F, C7F2); - 119.54 (s 2F, C6F2).
Conclusions
It was shown that the alkylation of maleopimaric acid with 6,6,7,7-tetrafluoro-4-oxahept-1-ene, 6,6,7,7,8,8,9,9-octafluoro-4-oxanon-1-ene and 6,6,7,7,8,8,9,9,10,10,11,11-dodecafluoro-4-oxaundec-1-ene without using any solvent (or in toluene) in the presence of conc.H2SO4 at 104-158 °C for 20 h. leads to formation of the corresponding esters
The similar result was obtained by the reaction of 3- (1,1,2,2,3,3,4,4-octafluoropentoxy) propylabietate (III b) with maleic anhydride without using any solvent in the presence of conc. H2SO4 at 147-152°C for 2 h (yield 89%).
References
- Simonsen J. The terpenes. Volume III. The sesquiterpenes, diterpenes and derivatives. Cambridge: At the University press, 1952. 579 p.
- Sandermann W. Naturharze Terpentinöltallöl. Cheme und Technologie / Sprinder Verlag: Berlin, 1960. 172 p.
- Tolstikov G.A., Tolstikova T.G., Shul'c E.E., Tolstikov S.E., Hvostov M.V. Smolyanye kisloty hvojnyh Rossii. Novosibirsk: Akadem. Izd-vo «Geo», 2011. 396 s.
- Rosin – based Chemicals and Polymers / Ed. by Jinwen Zhang. Shawbury, Shewsbury, Shropshire: Smithers Rapra Technology Ltd., 2012. 234 p.
- Ishikawa N., Коbaeashi Y. Fluorine compounds. Chemistry and application. М.: Мir, 1982. 276 p.
- New of Technology of Fluorine Compounds / Red. Ishikawa N. М.: Мir, 1984. 592 p.
- Soedineniya ftora: Sintez i primenenie: per.s yap. // pod red. N.Isikavy. M.: Mir, 1990. 407 s.
- Popova L.M., Vershilov S.V. Acetoksilirovanie 6,6,7,7,8,8,9,9-oktaftorpentoksipropena-1 smolyanymi kislotami. Fluorine Notes. 2015. N 2.
- M. Hudlicky. Chemistry of organic fluorine compounds. M.: Goschimizdat, 1961. 373 p.
- Kalninysh A.I., Dobelis Yu.Ya. Dienovyj sintez v oblasti smolyanyh kislot. Primenenie katalizatorov pri poluchenii adduktov smolyanyh kislot s dienofilami. Izv. AN Latv.SSR. 1969. №5. S. 54-57.
Recommended for publication by V. Kornilov
Fluorine Notes, 2018, 118, 3-4
