Received: April 2017
DOI 10.17677/fn20714807.2017.02.02
Fluorine Notes, 2017, 111, 3-4
NMR Spectral Characteristics of Fluorocontaining Pyridines
Pospelova N. B. 1, Mokrushin I. G. 2
1Federal State Unitary Enterprise "Russian Scientific Center" Applied Chemistry",
2Perm State National Research University, Perm, Russia
Abstract: Nuclear magnetic resonance spectroscopy of fluorinated pyridines - a convenient method of identification and the analysis. In article the received information on chemical shifts and constants of signals of kernels of hydrogen, carbon-13, nitrogen-15, fluorine-19 is collected. Conclusions on dependence of position of a signal on experimental conditions and structure substituted pyridines are drawn.
Keywords: nuclear magnetic resonance, NMR, fluorine-19, perfluorinated compounds, fluoropyridines.
Introduction
From the beginning of 1960-s the development of synthesis methods of fluorine containing compounds of pharmacological and medical and biological properties had been going on with growing intensity. The obtaining of any, even completely fluorinated alkaloid is possible in theory at an up-to-date level. The examples of fluorine containing compounds include with astonishing activity compound I (Diflucan, Fluconazol) used for curing mycosis and candidosis, compunds II and III with antibacterial characteristics even if the resistance to other medicines had been formed compounds IV and V insecticides inhibitors of quinine used in agriculture.
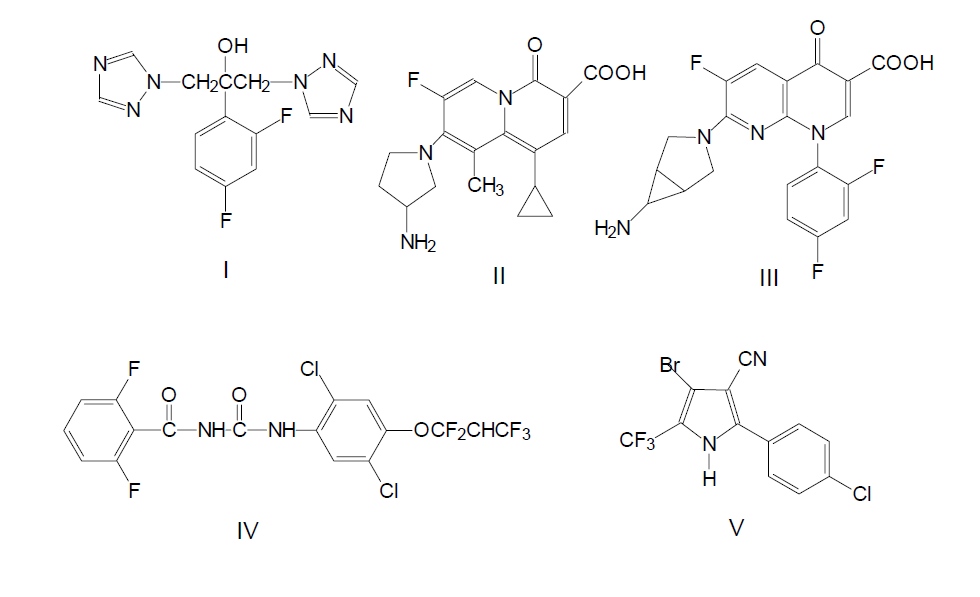
Pyridine and its derivatives form the base of a great number of alkaloids. The modification of natural systems and introduction of fluorine atoms into their composition can lead to increasing of the biological activity of such compounds, serious decreasing of ability to participate in metabolism, increasing of specificity and connectivity by receptors sites.
We have described NMR spectral characteristics of a number of fluorine containing pyridines synthesized at FSUE RSC Applied Chemistry and Perm Chemical Company LLC. H1, F19, C13 and N15 spectra of NMR have been obtained. Some literature data for substituted pyridines were also included into this publication.
Factors Influencing Chemical Shifts
The main factors defining the position of signal in spectrum are local diamagnetic and paramagnetic shielding caused by movement of own orbital atoms of electron, internuclear quadrupolar (as system of dipoles) and spin-spin coupling (between spin magnetic moments), influence of neighboring atoms and electrical fields from charged or polar groups (for example, when forming hydrogen bonds or solvation).
Pyridine’s molecule is polarized and the negative polarization centre is concentrated at nitrogen atom. Atoms of big electron density around core (diamagnetic component) and low excitation energy of outer orbitals (paramagnetic component) influence the most the chemical shift of protons. Van Vleck paramagnetism is a deformation of electron shell using applied magnetic field with appearance of additional magnetic moment.
The size of diamagnetic shielding depends significantly on electronic density around core: the higher the density, the bigger the shielding and the smaller the chemical shift is. Based on data on substituent electronegativity parameters the prediction of corresponding signal position area in spectrum is possible. Number of examples of good correlation between proton chemical shift and substituent electronegativity parameters in methane has been described. It has been noted, that C-Hal bond magnetic anisotropy additionally decreases protons shielding compare to non-halogens analogous in electronegativity (for example, chalcogens) [1, 2].
Solvent Impact On Chemical Shift And Spectrum Type
“The real” parameter of chemical shift can be obtained by recording of infinitely dilute solution. In practice, we use 5-10% (weight) concentration of sample to take off the exchange interactions.
The choice of solvent to obtain NMR spectra of fluoroorganic compounds is matter of importance. A number of researchers, for example, P. V. Nikul’shin shows chemical shifts of substituted pyridines dissolved in CCl4 with addition of ACETONE-D6 or CDCl3. For the purpose of studying the impact of solvent on signals chemical shift the spectra of monofluor-, difluor- and tetrafluoropyridine were registered in a pure form with capillary and in the form of solutions in chloroform and acetone. We can calibrate the spectrum of pure liquid sample using standard in deuterated solvent placed into glass capillary. This economical method simplifies the obtaining of spectrum and doesn’t contaminate the sample.
According to literature data the direct constants 1J (C, F) are within the range of -150÷-300 Hz and the solvent used influences them in many cases [3]. Geminal constants 2J (C, F) are always positive. Usually, they are within the range of 10÷30 Hz. Below you will find absolute values of constants.
It was found, that dissolving of sample in chloroform shifted signals (in relation to clean sample recorded with outer capillary with DMSO-d6/benzotrifluoride) towards weaker field: proton one – for 5-9% (0,4-0,7 ppm), fluorine one – for 1,5-6,5% (1-6 ppm), carbon one – for 1-2% (1-3 ppm).
Dissolving of the same sample in acetone increases the difference in chemical shift twice in most cases (re ΔCDCl3-pure) – 13С for 1,8-3% (2-4 ppm) and 1H for 3-12% (0,3-1 ppm), towards weak filed, however it influences at a significantly less scale the position of lines 19F Δ = 0,1-4,5% (1-4 ppm).
Spin-spin coupling constants J (C, F) и J (F, F) depend insignificantly on the conditions of spectrum recording. Solvent and sample concentration do not influence the form of all the cores under study. The range of values caused to a more extent by casual factors amounts to 1-5% (up to 4 Hz). The presence of solvent doesn’t influence “the inverse roof effect” too, e.g. the alteration of relative intensity of lines in multiplet due to mutual impact of energy states of interacting cores – the dipole-dipole interaction result appearing in 13С spectra of substituted pyridines in a number of cases.
Results
Data for solutions of pyridines in d-chloroform can be found in tables, literature data are listed “as they are” with references to a source. The absence of value of chemical shift in corresponding box tells us, that spectrum had not been recorded due to different reasons. The values of δ NMR chemical shifts of 15N nitrogen of hydrogen containing pyridines were obtained from 2D 1H-15N HMBC (Heteronuclear Multiple Bond Correlation) experiments.
Spin-spin coupling constant – (JFF) of substituted pyridines are decreasing as the distance between them is growing. JCF constants are growing as pyridine is being fluorinated and is getting away along the chain from nitrogen atom, thus JC2F = 236 Hz, and JC4F = 265 Hz.
Table 1. Found Ranges of Constants of Spin-Spin Coupling J, Hz:
|
F2-F3, F5-F6 |
F2-F4, F4-F6 |
F2-F5, F3-F6 |
F2-F6 |
F3-F4, F4-F5 |
C-F |
C-CF |
C-С-CF |
C6-C2F |
C5-C2F |
|
20-32 |
18-20 |
20-24 |
12-13 |
17-18 |
230-270 |
20-40 |
6-9 |
13-15 |
3-5 |
1. Perfluoropyridine and tetrafluoropyridines
Chemical shift of fluorine in α, β and γ positions differs dramatically. Fluorine is unscreened in α-position to a most extent, and it is mostly screened in β-position.
The value of electronegativity of tetrafluorpyridine para-substituent influences the chemical shift of fluorine in ortho-position. F2,6 signal is descreened and shifted towards weaker field as electronegativity grows in a row of H<CH2COOH<Br<Cl<CN<F, as internuclear quadrupole and spin-spin coupling with para-substituent influences F3,5 signal to a greater extent, for example, in 4-bromine and 4-cianotetrafluoropyridine.
|
Molecular Formula |
α-F |
β-F |
γ-R |
Solvent |
P.S. |
|
C5F5N* |
-87.8 |
-161.2 |
-133.5 |
ACETONE-D6 |
- |
|
C5F5N |
-87.4 |
-161.6 |
-133.6 |
CDCl3 |
- |
|
C5F4NH |
-94.0 |
-142.1 |
7.17 |
CDCl3 |
15N: -126 |
|
C5F4NBr |
-88.5 |
-135.3 |
Br |
CCl4 |
[4] |
|
C5F4NCl* |
-89.5 |
-142.1 |
Cl |
ACETONE-D6 |
- |
|
C6F4N2* |
-87.7 |
-134.3 |
CN |
ACETONE-D6 |
- |
|
C7F4NH3* |
-92.5 |
-143.6 |
CH2COOH |
ACETONE-D6 |
- |
*The spectrum was recorded by N.B. Pospelova using Bruker WP80SY.
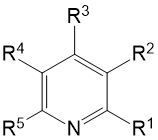
|
Molecular Formula |
R1 |
R2 |
R3 |
R4 |
R5 |
Solvent |
P.S. |
|
C5F4NH |
-90.3 |
-164.5 |
-150.7 |
-155.8 |
7,17 |
ACETONE-D6 |
[6] |
|
C5F4NCl |
-85.6 |
-163.6 |
-113.9 |
Cl |
-72.2 |
CDCl3 |
15N: -136 |
2. Trifluoropyridines
|
Molecular Formula |
R1 |
R2 |
R3 |
R4 |
R5 |
Solvent |
P.S. |
|
C5F3NCl2 |
-87.5 |
-141.6 |
Cl |
Cl |
-71.3 |
CCl4 |
[4] |
|
C5F3NH2 |
-90.8 |
-135.4 |
7.37 |
-128.6 |
7.82 |
CDCl3 |
15N: -95 |
|
C5F3NH2 |
-88.6 |
-147.6 |
7.69 |
6.80 |
-74.7 |
CDCl3 |
15N: -95 |
|
C5F3NH2 |
-83 |
-165 |
-123 |
H |
H |
- |
[5] |
|
C5F3NH2 |
-91 |
-136 |
7.6 |
-129 |
7.92 |
- |
[5] |
|
C5F3NH2 |
-86 |
-147 |
7.63 |
6.75 |
-73 |
- |
[5] |
|
C5F3NH2 |
-65 |
6.54 |
-95 |
6.54 |
-65 |
- |
[5] |
|
C5F3NH2 |
H |
-146 |
-148 |
-146 |
H |
- |
[5] |
|
C5F3NHCl |
-89.4 |
-143.01 |
Cl |
7.79 |
-76.4 |
CDCl3 |
15N: -75 |
|
C5F3NHClS |
-90.1 |
-139.6 |
SH |
Cl |
-73.9 |
CCl4 |
[4] |
|
C6F3NH2O2* |
COOH |
-140.97 |
7.75 |
-140.3 |
-94.64 |
ACETONE-D6 |
COOH: 10.40 |
|
C5F3NHCl |
-85.1 |
-149 |
Cl |
H |
-72.1 |
pure |
[6] |
|
C5F3N2H2Cl* |
NH2 |
-159.6 |
Cl |
-144.07 |
-91.75 |
ACETONE-D6 |
NH2: 5.96 |
|
C5F3N2H3 |
-95 |
-173.7 |
NH2 |
H |
-76.4 |
ACETONE-D6 |
[6] |
|
C5F3N2H3 |
NH2 |
-124 |
-172.6 |
H |
-70.9 |
ACETONE-D6 |
[6] |
*The spectrum was recorded by N.B. Pospelova using Bruker WP80SY.
3. Difluoropyridines
|
R1 |
R2 |
R3 |
R4 |
R5 |
Solvent |
P.S. |
|
|
C5F2NCl3 |
-68.94 |
Cl |
Cl |
Cl |
-68.94 |
pure |
- |
|
C5F2NH3 |
8.37 |
-124 |
7.2 |
-124 |
8.37 |
- |
[5] |
|
C5F2NH3 |
-87 |
-140 |
7.75 |
7.16 |
7.91 |
- |
[5] |
|
C5F2NH3 |
-63 |
6.64 |
-99 |
6.95 |
8.2 |
- |
[5] |
|
C5F2NH3 |
-73 |
6.86 |
7.48 |
-134 |
7.98 |
- |
[5] |
|
C5F2NH3 |
-70 |
6.73 |
7.8 |
6.73 |
-70 |
- |
[5] |
|
C5F2NH3 |
H |
-153 |
-130 |
H |
H |
- |
[5] |
|
C5F2NH3 |
-69.5 |
6.68 |
7.79 |
6.68 |
-69.5 |
CDCl3 |
15N: -133 |
|
C5F2NHCl2 |
-73.04 |
Cl |
7.95 |
Cl |
-73.04 |
CDCl3 |
15N: -131 |
|
C5F2N2H3Cl |
NH2 |
-150.7 |
Cl |
H |
-74.6 |
ACETONE-D6 |
[6] |
|
C5F2N2H4 |
-68.7 |
H |
NH2 |
H |
-68.7 |
ACETONE-D6 |
[7] |
-
- Fluoropyridines
|
R1 |
R2 |
R3 |
R4 |
R5 |
Solvent |
P.S. |
|
|
C5FN2H3Cl2* |
NH2 |
Cl |
7.66 |
Cl |
-75.87 |
ACETONE-D6 |
NH2: 5.33 |
|
C5FNH4 |
-68 |
6.9 |
7.8 |
7.2 |
8.2 |
- |
[5] |
|
C5FNH4 |
-68.35 |
6.79 |
7.66 |
7.05 |
8.09 |
CDCl3 |
15N: -103 |
|
C5FNH5 |
H |
-126 |
H |
H |
H |
- |
[5] |
|
C5FNH6 |
H |
H |
-103 |
H |
H |
- |
[5] |
|
C5FNH7+HCl |
-79 |
H |
H |
H |
H |
- |
[5] |
|
C5FNH8+HCl |
H |
-114 |
H |
H |
H |
- |
[5] |
|
C6FNH5OCl* |
7.44 |
Cl |
7.44 |
-136.23 |
OCH3 |
ACETONE-D6 |
OCH3: 3.96 |
*The spectrum was recorded by N. B. Pospelova using WP80SY
Experimental
The authors would like to thank PhD P. V. Podsevalov for samples kindly provided.
NMR spectra of substituted pyridines were recorded using Bruker WP80SY 80 MHz and Avance III HD 400 MHz. Chemical shifts are listed in parts per million (ppm). The signal of solvent is taken as standard in H1NMR spectra(δ, ppm, re TMS):2.05- acetone-D6; 2.50 - DMSO-D6, 7.26 - CDCl3; in spectra 13C (ppm, re TMS): 29.84 - acetone-D6; 39.52 - DMSO-D6, 77.16 - CDCl3; in 19F spectra the signal of benzotrifluoride - -63.70 ppm, re CFCl3; in 15N (1H-15N HMBC) spectra the signal of nitromethane 0.0 ppm;
Pyridine. 1H (pure liquid) δ 8.23 – 8.08 (m, 2H, С2,4H), 7.04 (tt, J = 7.7, 1.9 Hz, 1H, С4H), 6.72 – 6.58 (m, 2H, С3,5H). 13C (pure liquid) δ 148.89, 134.72, 122.72. 1H (CDCl3) δ 7.98 (dt, J = 3.7, 1.7 Hz, 2H, С2,4H), 6.95 (tt, J = 7.6, 1.8 Hz, 1H, С4H), 6.57 (ddd, J = 7.7, 4.3, 1.6 Hz, 2H, С3,5H). 13C (CDCl3) δ 148.62, 134.63, 122.52. 15N (CDCl3) δ -63 (s).
Pentafluoropyridine. 19F (pure liquid) δ -93.19 (dm, J = 14.3 Hz), -139.42 (tt, J = 16.6, 14.0 Hz), -167.40 (m). 13C (pure liquid) δ 148.02 (dtt, J = 267.7, 12.3, 6.2 Hz), 142.31 (ddddd, J = 244.1, 16.4, 13,4, 5.6, 2.8 Hz), 131.97 (dm, J = 209.7 Hz). 13C (CDCl3) δ 149.68 (dtt, J = 268.1, 12.2, 6.1 Hz), 144.13 (dm, J = 244.9 Hz), 133.71 (dm, J = 291.2 Hz). 19F (CDCl3) δ -87.37 (d, J = 12.8 Hz), -133.56 (tt, J = 17.6, 13.8 Hz), -161.62 (m). 13C (Ac-d6) δ 150.97 (dtt, J = 266.0, 12.4, 6.3 Hz), 145.20 (d, J = 242.2 Hz), 135.09 (d, J = 259.8 Hz). 19F (Ac-d6) δ -89.28 (dm, J = 12.1 Hz), -135.08 (tt, J = 17.4, 13.9 Hz), -162.9 (m).
2,3,5,6-Tetrafluoropyridine. 1H (pure liquid) δ 7.23 – 7.11 (7.17) (m, 1H). 19F (pure liquid) δ -93.69 (dt, J = 28.3, 12.8 Hz), -141.84 – -142.10 (-142.27) (m). 13C (pure liquid) δ 142.00 (dddd, J = 16.3, 15.1, 12.3, 2.3 Hz), 142.1-139.0 (140.54) (dm, J = 265.1 Hz), 117.56 (tt, J = 21.1, 3.1 Hz). 1H (CDCl3) δ 7.63 – 7.55 (7.59) (m, 1H). 19F (CDCl3) δ -91.96 (dt, J = 27.5, 13.3 Hz), -140.65 – -140.87 (-140.06) (m). 13C (CDCl3) δ 143.24 (dddd, J = 17.0, 15.6, 12.1, 2.2 Hz), 142.9-139.8 (141.35) (dm, J = 264 Hz), 121.26 (tt, J = 21.2, 3.0 Hz). 15N (CDCl3) δ -126 (s). 1H (Ac-d6) δ 8.18 (tt, J = 8.1, 7.1 Hz, 1H). 19F (Ac-d6) δ -94.00 (td, J = 28.8, 13.6 Hz), -141.91 – -142.11 (-142.31) (m). 13C (Ac-d6) δ 144.56 (dddd, J = 16.8, 15.5, 12.0, 2.2 Hz), 144.7-141.6 (143.17) (dm, J = 263.3 Hz), 121.09 (tt, J = 21.3, 3.0 Hz).
2-Fluoropyridine. 1H (pure liquid) δ 7.66-7.57 (7.62) (dm, J = 4.9 Hz, 1H), 7.19 (tdd, J = 8.3, 7.2, 2.1 Hz, 1H), 6.57 (dddd, J = 7.3, 4.9, 2.5, 0.9 Hz, 1H), 6.35 (ddt, J = 8.3, 2.7, 0.8 Hz, 1H). 13C (pure liquid) δ 162.56 (d, J = 236.6 Hz), 146.46 (d, J = 14.8 Hz), 140.05 (d, J = 7.8 Hz), 120.22 (d, J = 4.1 Hz), 108.22 (d, J = 37.5 Hz). 19F (pure liquid) δ -71.32 (s). 1H (CDCl3) δ 8.12-8.06 (8.09) (dm, J = 4.9 Hz, 1H), 7.66 (tdd, J = 8.2, 7.2, 2.1 Hz, 1H), 7.05 (dddd, J = 7.3, 4.9, 2.4, 0.9 Hz, 1H), 6.79 (ddt, J = 8.3, 2.7, 0.7 Hz, 1H). 13C (CDCl3) δ 163.57 (d, J = 238.4 Hz), 147.61 (d, J = 14.5 Hz), 140.96 (d, J = 7.8 Hz), 121.10 (d, J = 4.2 Hz), 109.46 (d, J = 37.0 Hz). 19F (CDCl3) δ -68.35 (s). 15N (CDCl3) δ -103 (s). 1H (Ac-d6) δ 8.57-8.21 (8.24) (dm, J = 4.7 Hz, 1H), 7.94 (tdd, J = 8.3, 7.2, 2.1 Hz, 1H), 7.30 (dddd, J = 7.4, 4.9, 2.6, 0.8 Hz, 1H), 7.05 (ddt, J = 8.3, 2.7, 0.8 Hz, 1H). 13C (Ac-d6) δ 164.63 (d, J = 235.9 Hz), 148.69 (d, J = 15.0 Hz), 142.42 (d, J = 7.8 Hz), 122.45 (d, J = 4.2 Hz), 110.30 (d, J = 37.6 Hz). 19F (Ac-d6) δ -69.56 (s).
2,3,5-Triluoropyridine. 1H (pure liquid) δ 7.26 (t, J = 2.5 Hz, 1H), 6.99 (dtd, J = 9.0, 7.3, 2.6 Hz, 1H). 13C (pure liquid) δ 155.70 (ddd, J = 255.7, 4.0, 2.7 Hz), 146.79 (ddd, J = 234.2, 14.3, 2.0 Hz), 143.61 (ddd, J = 265.5, 32.2, 7.6 Hz), 126.98 (ddd, J = 27.0, 14.2, 5.8 Hz), 113.84 (ddd, J = 24.8, 18.3, 3.6 Hz). 19F (pure liquid) δ -91.14 (t, J = 27.1 Hz), -128.48 (dd, J = 29.2, 3.2 Hz), -135.60 (dd, J = 25.7, 3.3 Hz). 1H (CDCl3) δ 7.77 (t, J = 2.5 Hz, C6H), 7.37 (dtd, J = 8.7, 7.2, 2.6 Hz, C4H). 13C NMR (CDCl3) δ 156.85 (ddd, J = 256.4, 4.0, 2.7 Hz), 148.16 (ddd, J = 235.1, 14.2, 1.7 Hz), 144.93 (ddd, J = 266.1, 32.0, 7.4 Hz), 128.56 (ddd, J = 26.9, 13.9, 5.8 Hz), 115.38 (ddd, J = 24.5, 18.1, 3.6 Hz). 19F (CDCl3) δ -90.81 (t, J = 26.9 Hz), -128.55 (dd, J = 29.1, 3.2 Hz), -135.39 (dd, J = 25.6, 3.2 Hz). 15N (CDCl3) δ -95 (s).
2,3,6-три Triluoropyridine. 1H (CDCl3) δ 7.69 (dddd, J = 9.1, 8.6, 8.1, 6.0 Hz, 1H), 6.80 (ddd, J = 8.6, 3.3, 2.2 Hz, 1H). 13C (CDCl3) δ 155.61 (dd, J = 244.1, 11.3 Hz), 151.12 – 147.06 (m), 142.99 (ddd, J = 253.7, 25.1, 6.4 Hz), 131.33 (ddd, J = 18.4, 8.5, 2.9 Hz), 106.89 (ddd, J = 38.5, 6.2, 2.9 Hz). 19F (CDCl3) δ -74.66 (dd, J = 24.5, 10.3 Hz), -88.61 (dd, J = 17.0, 12.5 Hz), -147.58 (dd, J = 26.9, 21.8 Hz). 15N (CDCl3) δ -95 (s).
2,6-Difluoro-3,5-dichloropyridine. 1H (Ac-d6) δ 8.42 (t, J = 7.7 Hz, 1H). 13C (Ac-d6) δ 155.48 (dd, J = 244.5, 13.4 Hz), 145.75 (t, J = 1.8 Hz), 114.57 (dd, J = 22.2, 13.4 Hz), 114.57 (d, J = 40.2 Hz). 19F (Ac-d6) δ -75.49 (s). 1H (CDCl3) δ 7.95 (t, J = 7.5 Hz, 1H). 13C (CDCl3) δ 154.81 (dd, J = 247.7, 13.0 Hz), 143.99 (t, J = 1.8 Hz), 113.95 (dd, J = 22.1, 13.0 Hz), 113.95 (d, J = 40.2 Hz). 19F (CDCl3) δ -73.04 (s). 15N (CDCl3) δ -131 (s).
2,6-Difluoropyridine. 1H (pure liquid) δ 7.90 (p, J = 8.0 Hz, 1H), 6.80 (d, J = 8.0 Hz, 2H). 13C (pure liquid) δ 160.38 (dd, J = 245.3, 14.9 Hz), 144.23 (t, J = 7.8 Hz), 106.38 – 103.15 (m). 19F (pure liquid) δ -72.94 (s). 1H (Ac-d6) δ 8.14 (p, J = 8.1 Hz, 1H), 7.02 (d, J = 8.0 Hz, 2H). 13C (Ac-d6) δ 162.54 (dd, J = 244.6, 15.0 Hz), 147.12 (t, J = 7.8 Hz), 107.34 – 106.64 (m).19F (Ac-d6) δ -70.63 (s). 1H (CDCl3) δ 7.79 (p, J = 8.0 Hz, 1H), 6.68 (d, J = 8.0 Hz, 2H). 13C (CDCl3) δ 161.52 (dd, J = 246.1, 14.8 Hz), 145.20 (t, J = 7.7 Hz), 109.14 – 101.15 (m). 19F (CDCl3) δ -69.48 (s). 15N (CDCl3) δ -133 (s).
3-Chloro-2,5,6-trifluoropyridine. 1H (pure liquid) δ 7.13 (td, J = 7.6, 6.8 Hz, 1H). 13C (pure liquid) δ 149.35 (ddd, J = 243.2, 11.5, 2.4 Hz), 145.63 (ddd, J = 247.0, 16.8, 12.5 Hz), 140.96 (ddd, J = 259.9, 27.1, 6.3 Hz), 129.69 (ddd, J = 20.3, 3.0, 2.0 Hz), 111.52 (ddd, J = 35.3, 6.6, 3.1 Hz). 19F (pure liquid) δ -80.36 (dd, J = 28.0, 11.4 Hz), -93.39 (dd, J = 20.1, 12.0 Hz), -146.69 (dd, J = 28.2, 20.9 Hz). 1H (CDCl3) δ 7.80 (td, J = 7.6, 6.8 Hz, 1H). 13C (CDCl3) δ 151.08 (ddd, J = 243.7, 11.4, 2.4 Hz), 147.32 (ddd, J = 247.4, 16.7, 12.4 Hz), 142.57 (ddd, J = 260.1, 27.0, 6.2 Hz), 131.36 (ddd, J = 20.0, 3.1, 2.0 Hz), 113.22 (ddd, J = 35.3, 6.6, 3.0 Hz). 19F (CDCl3) δ -76.44 (dd, J = 27.7, 10.9 Hz), -89.41 (dd, J = 19.5, 11.8 Hz), -142.99 (dd, J = 28.1, 21.1 Hz). 15N (CDCl3) δ -75.5 (s).
5- Chloro -2,3,4,6-tetrafluoropyridine. 13C (pure liquid) δ 155.11 (ddt, J = 266.2, 11.5, 5.8 Hz), 149.52 (dddd, J = 243.0, 15.0, 5.8, 3.4 Hz), 146.07 (dddd, J = 245.5, 16.1, 13.4, 6.7 Hz), 131.81 (dddd, J = 263.1, 30.0, 14.3, 8.1 Hz), 102.29 (dddd, J = 38.5, 17.7, 8.0, 2.9 Hz). 19F (pure liquid) δ -76.90 (dt, J = 23.2, 11.8 Hz), -90.45 (dd, J = 33.9, 17.4 Hz), -118.88 (td, J = 17.6, 10.6 Hz), -168.48 (ddd, J = 24.7, 20.3, 17.5 Hz). 13C (CDCl3) δ 156.96 (ddt, J = 266.4, 11.5, 5.8 Hz), 151.49 (dddd, J = 243.5, 14.9, 5.6, 3.3 Hz), 147.99 (dddd, J = 246.0, 16.0, 13.3, 6.6 Hz), 133.70 (dddd, J = 263.4, 29.9, 14.3, 8.1 Hz), 104.33 (dddd, J = 38.3, 17.7, 8.0, 2.8 Hz). 19F (CDCl3) δ -71.97 – -72.20 (m), -85.60 (dd, J = 32.8, 16.4 Hz), -113.93 (td, J = 17.9, 10.5 Hz), -163.61 (ddd, J = 24.7, 20.6, 18.1 Hz).
2,3,4,6- Tetrafluoropyridine. 1H (pure liquid) δ 6.27 (dm, J = 8.5 Hz, 1H). 13C (pure liquid) δ 159.03 (dddd, J = 265.2, 13.2, 10.4, 5.7 Hz), 153.97 (dtd, J = 243.2, 15.7, 3.7 Hz), 148.80 (dddd, J = 243.1, 18.8, 13.1, 6.8 Hz), 131.78 (dddd, J = 257.2, 28.5, 14.0, 8.6 Hz), 95.31 (dddd, J = 42.9, 21.0, 6.8, 2.4 Hz). 19F (pure liquid) δ -75.02 (dd, J = 31.5, 13.3 Hz), -91.54 (dd, J = 33.6, 17.4 Hz), -120.79 (ddd, J = 18.9, 17.7, 15.1 Hz), -175.67 (ddd, J = 22.4, 20.9, 17.8 Hz). 1H (CDCl3) δ 6.73 (dddd, J = 8.3, 3.7, 2.8, 1.6 Hz, 1H). 13C (CDCl3) δ 160.54 (dddd, J = 265.6, 13.1, 10.4, 5.7 Hz), 155.51 (dtd, J = 243.7, 15.6, 3.7 Hz), 133.40 (dddd, J = 257.6, 28.2, 14.0, 8.5 Hz), 97.11 (dddd, J = 42.5, 20.7, 6.8, 2.4 Hz). 19F (CDCl3) δ -69.38 (dd, J = 29.4, 15.6 Hz), -85.58 (dd, J = 32.5, 16.4 Hz), -115.04 (td, J = 18.7, 15.2 Hz), -169.82 (ddd, J = 22.7, 21.0, 18.3 Hz). 15N (CDCl3) δ -136 (s).
Difluorotrichloropyridine. 13C (pure liquid) δ 152.90 (dd, J = 247.5, 15.7 Hz), 145.20 (t, J = 3.0 Hz), 112.76 – 111.85 (m). 19F (pure liquid) δ -68.94 (s).
Tetrafluoropyridine. 1H (CDCl3) δ 7.87 (s, J = 1.2 Hz, 1H). 13C (CDCl3) δ 146.17 (s), 140.30 (s), 129.82 (s). 15N (CDCl3) δ -50.5 (s).
References
- Основы ядерного магнитного резонанса: Учебное пособие / М.П. Евстигнеев, А.О. Лантушенко, В. В. Костюков. и др. - М.: Вузовский учебник, НИЦ ИНФРА-М, 2015. – 247 с.
- Днепровский А.С., Темникова Т.И. Теоретические основы органической химии, Л: Химия, 1991. – 558 с.
- Воловенко Ю.М., Карцев В.Г., Комаров И.В., Туров А.В., Хиля В.П., Спектроскопия ядерногомагнитного резонанса для химиков, М.: МБФНП, 2011. – 694 с.
- Никульшин П. В. Изучение термических реакций полифторарентиолов с хлором, бромом и их источниками. Дисс. канд. хим. наук: 02.00.03: 07.10.2016 / Никульшин Павел Викторович. – Новосибирск, 2016. – 151 с.
- Dolbier, William R. Guide to Fluorine NMR for Organic Chemists. John Wiley & Sons, 2006. – 235 p.
- Richard D. Chambers, John S. Waterhouse, and D. Lyn H. Williams. Mechanisms for Reactions of Halogenated Compounds. Part 1. Activating Effects of Fluorine in Polyfluoropyridines in Reactions with Ammonia // J. Chem. Soc., Perkin Trans. 2, 1977, 585-588.
- Chambers RD, Seabury MJ, Williams D, Hughes N. Mechanisms for reactions of halogenated compounds. Part 6. Investigations into the activating effect of ortho-fluorine in nucleophilic aromatic substitution // J Chem Soc Perkin Trans. 1, 1988: 255–258.
- J.-E. Backvall, J.E. Baldwin, R.M. Williams. High-Resolution NMR Techniques in Organic Chemistry, Elsevier, 2009.
- Gerig, J. T., Fluorine, N. M. R., Chapter in On-line Textbook, Biophysical Society, 2001 (www.biophysics.org/img/jtg2001-2.pdf).
Recommended for publication by Prof. S. Igumnov
Fluorine Notes, 2017, 111, 3-4
