Received: August 2017
DOI 10.17677/fn20714807.2017.05.01
Fluorine Notes, 2017, 114, 1-2
Synthesis of Sterically Hindered Fluorous Aryl Perfluoroalkyl Sulfides
Antal Harsányi, Gitta Schlosser and József Rábai*
Institute of Chemistry, Eötvös Loránd University, Pázmány Péter sétány 1-A, Budapest, H-1117, Hungary
e-mail: rabai@elte.hu
Abstract: The sodium salt of 2,6-dimethyl-4-tert-butyl-benzenethiol was reacted in dimethyl formamide with a series of perfluoroalkyl iodides and 1,8-diiodoperfluorooctane to afford the corresponding perfluoroalkyl sulfides and 1,8-bis(arylthio)perfluorooctane in good yields.
Keywords: fluorous sulfides, perfluoroalkyl iodides, perfluoroalkylation
Inspired by the introduction of FluoleadTM by Umemoto et al. [1] as a novel fluorinating reagent and with the early publication on the preparation of some aryl(trifluoromethyl)difluorosulfuranes by Yagupolskii et al. [2] we aimed at to synthesize ArSF2Rfn type sulfuranes (4) (Scheme 1).
Such perfluoroalkyl substituted reagents are expected to have unique physical-chemical properties similar to that of fluorocarbons and allowing easy separation of used reagents from products [3].
Here we disclose the optimized synthesis of precursor aryl perfluoroalkyl sulfides (3a-e) based on spontaneous perfluoroalkylation of thiols without initiators under similar conditions that reported for simple thiols by Feiring and Boiko [4](Scheme 1).
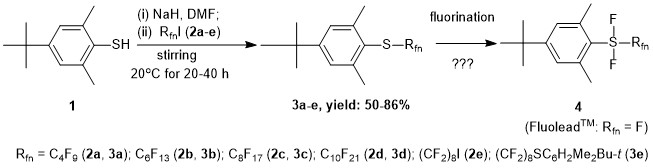
Scheme 1. Planned synthesis of novel fluorous aryl perfluoroalkyl difluorosulfuranes 4.
Sterically hindered aryl perfluoroalkyl sulfides 3a-e were prepared in good to excellent isolated yields using the reaction of the sodium salt of 1 with a slight excess of 1-iodoperfluoroalkanes (RfnI, 2a-d) or with that of 1,8-diiodoperfluorooctane (2e) in absolute DMF at room temperature for 20 to 40 h. The purified new fluorous sulfides 3a-e were appropriately characterized. It is worth to mention that mass spectrometric measurements by APCI technique were highly facilitated by using a 1:1 vol/vol solvent mixture of CH3CN and CF3CH2OH for sample preparation. The synthesis of the analogue trifluoromethyl sulfide (Rfn = CF3) was effected by using CF3I dissolved in DMF as a perfluoroalkylating reagent and reported earlier by us [5].
Our attempts for oxidative fluorination of 3 to 4 (Rfn = CF3) with the use of Br2/KF/CH2Cl2 and some other reagent systems however has not succeeded yet [6].
Experimental
1H-, 13C- and 19F-NMR spectra were recorded on Bruker Avance 250 instrument using a 5 mm inverse 1H/13C/31P/19F probe head at room temperature. Chemical shifts (δ) are given in parts per million (ppm) units relatively solvent (CDCl3) residual peaks (δ=7.26 for 1H, δ=77.0 for 13C) and to CFCl3 as external standard (δ=0.00 for 19F). Determination of molecular mass was performed by atmospheric pressure chemical ionization mass spectrometry (APCI-MS) on a Bruker Daltonics Esquire 3000 plus (Germany) ion trap mass spectrometer. Samples were dissolved in acetonitrile – trifluoroethanol solvent mixture (50:50, V/V). Mass spectra were acquired in the 50-1500 m/z range yielding singly charged radical cations (M•+). Nebulizer gas pressure was 25 psi, drying gas flow was 5 L/min, the heated capillary temperature was 250 oC and the vaporizer temperature was 450 oC. Samples were injected into the ion source in a flow rate of 10 L/min using a syringe pump. Melting points were determined on a Böetius micro-melting point apparatus and are uncorrected. Gas chromatographic analysis of volatile products was performed using a Hewlett-Packard 5890 Series II instrument with PONA [crosslinked methylsilicone gum] 50 m x 0.2mm x 0.5 mm column, H2 carrier gas, FID detection; Program: 120 °C, 5 min, 10 °C/min, 250 °C, 5 min, Inj.: 250°C, Det.: 280°C.
General Procedure for the Synthesis of Aryl Perfluoroalkyl Sulfides (GP) [7]
4-(tert-Butyl)-2,6-dimethylbenzenethiol [8] (1.93 g, 10 mmol) was suspended in absolute DMF (15 mL) and reacted with sodium hydride (11 mmol) in small portions, prepared by washing under an argon atmosphere a 57% w/w sodium hydride – white oil dispersion with pentane (3 x 5 mL). When the evolution of hydrogen ceased the perfluoroalkyl iodide (2a-d, CnF2n+1I, [n=4,6,8,10], 11.0 mmol) or 1,8-diiodoperfluorooctane (2e, I(CF2)8I, 5.50 mmol) was added and the mixture was stirred at room temperature for 20 h (3a-c) or 40 h (3d-e) under an N2 atmosphere. Then the reaction mixture was poured into water (100 mL) and extracted with diethyl ether (3 x 20 mL), the combined organic extracts were washed with water (3 x 20 mL) and saturated aq-NaCl solution (20 mL). The ether phase was separated and dried (Na2SO4), then the ether was removed by distillation and the product was purified by vacuum distillation or crystallization.
(4-(tert-Butyl)-2,6-dimethylphenyl)(perfluorobutyl)sulfide (3a)
Yield: 2.90 g (71 %) colourless liquid, obtained by short path distillation; 20 Hgmm@160°C bath. It solidifies
in the freezer. GC assay: 98%+, tRET: 14.47 min. 1H NMR (250 MHz, CDCl3):
δ 1.33 (s, 9H, C(CH3)3), 2.57 (s, 6H, CH3), 7.22 (s, 2H, Ar CH).
13C NMR (62.5 MHz, CDCl3): δ 22.88, 31.43, 34.97, 118.81, 126.34, 145.98, 154.82.
19F NMR (243 MHz, CDCl3): δ -81,50 (m, 3F, CF3),
-85,96 (m,
2F, CF2), -121,29 (m, 2F, CF2), -126,01 (m, 2F, CF2). MS (APCI,
M•+): calcd. for C16H17F9S = 412.1; measured: 412.0.
(4-(tert-Butyl)-2,6-dimethylphenyl)(perfluorohexyl)sulfide (3b)
Yield: 3.50 g (68 %) white waxy solid with mp = 32-34 oC, obtained by short path distillation; 20 Hgmm@170°C bath. GC assay: 98%, tRET: 15.90 min. 1H NMR (250 MHz, CDCl3): δ 1.32 (s, 9H, C(CH3)3), 2.56 (s, 6H, CH3), 7.21 (s, 2H, Ar CH). 13C NMR (62.5 MHz, CDCl3): δ 22.89, 31.44, 34.97, 118.81, 126.34, 145.99, 154.82. 19F NMR (243 MHz, CDCl3): δ -81.33 (m, 3F, CF3), -85.74 (m, 2F, CF2), -120.38 (m, 2F, CF2), -121.85 (m, 2F, CF2), -123.28 (m, 2F, CF2), -126.63 (m, 2F, CF2). MS (APCI, M•+): calcd. for C18H17F13S = 512.1; measured: 511.9.
(4-(tert-Butyl)-2,6-dimethylphenyl)(perfluorooctyl)sulfide (3c)
Yield: 5.30 g (86 %) white crystals with mp = 53-54 oC, obtained by short path distillation; 0.5 Hgmm@120°C bath. GC assay: 98%, tRET: 17.31 min. 1H NMR (250 MHz, CDCl3): δ 1.31 (s, 9H, C(CH3)3), 2.55 (s, 6H, CH3), 7.20 (s, 2H, Ar CH). 13C NMR (62.5 MHz, CDCl3): δ 22.90, 31.45, 34.98, 118.82, 126.34, 145.98, 154.81. 19F NMR (243 MHz, CDCl3): δ -81.28 (m, 3F, CF3), -85.72 (m, 2F, CF2), -120.33 (m, 2F, CF2), -121.65 (m, 2F, CF2), -122.35 (m, 4F, CF2), -123.24 (m, 2F, CF2), -126.62 (m, 2F, CF2). MS (APCI, M•+): calcd. for C20H17F17S = 612.1; measured: 611.8.
(4-(tert-Butyl)-2,6-dimethylphenyl)(perfluorodecyl)sulfide (3d)
Yield: 5.70 g (76 %) white crystals with mp = 72-75 oC, obtained by short path distillation; 0.5 Hgmm@140°C bath. 1H NMR (250 MHz, CDCl3): δ 1.31 (s, 9H, C(CH3)3), 2.55 (s, 6H, CH3), 7.20 (s, 2H, Ar CH). 13C NMR (62,5 MHz, CDCl3): δ 22.90, 31.46, 34.98, 118.82, 126.33, 145.97, 154.81. 19F NMR (243 MHz, CDCl3): δ -81.26 (m, 3F, CF3), -85.72 (m, 2F, CF2), -120.33 (m, 2F, CF2), -121.64 (m, 2F, CF2), -122.24 (m, 8F, CF2), -123.20 (m, 2F, CF2), -126.58 (m, 2F, CF2). MS (APCI, M•+): calcd. for C22H17F21S = 712.1; measured: 711.8.
(Perfluorooctane-1,8-diyl)bis((4-(tert-butyl)-2,6-dimethylphenyl)sulfide (3e)
The crude product was recrystallization from acetone (15 mL). Yield: 2.80 g (50 %) white crystals with mp = 100-101 oC. 1H NMR (250 MHz, CDCl3): δ = 1.32 (s, 9H, C(CH3)3), 2.56 (s, 6H, CH3), 7.21 (s, 2H, Ar CH). 13C NMR (62.5 MHz, CDCl3): δ = 22.90; 31.45; 34.98; 118.82; 126.34; 145.98; 154.81. 19F NMR (243 MHz, CDCl3): δ = -85.67 (m, 4F, CF2), 120.31 (m, 4F, CF2), -121.62 (m, 4F, CF2), -122.22 (m, 4F, CF2). MS (APCI, M•+): calcd. for C32H34F16S2 = 786.2; measured: 786.0.
Acknowledgement
We thank the National Research, Development and Innovation Office for the financial support of the M-ERA.Net COR_ID program (NKFIH NN117633). G. S. acknowledges the support by the MTA János Bolyai Research Scholarship and by the MTA Premium Post-Doctorate Research Program of the Hungarian Academy of Sciences (HAS, MTA).
References
- Umemoto, T.; Singh, R. P.; Xu, Y.; Saito, N. Discovery of 4-tert-Butyl-2,6-dimethyl phenylsulfur Trifluoride as a Deoxofluorinating Agent with High Thermal Stability as Well as Unusual Resistance to Aqueous Hydrolysis, and Its Diverse Fluorination Capabilities Including Deoxofluoro-Arylsulfinylation with High Stereoselectivity. J. Am. Chem. Soc., 2010, 132, 18199–18205. DOI: 10.1021/ja106343h
- (a) Yagupolskii, L. M. Aromatic and Heterocyclic Compounds with Fluorine-Containing Substituents; Naukova Dumka: Kiev, USSR, 1988; (b) Yagupolskii, L. M.; Matsnev, A. V.; Orlova, R. K.; Deryabkin, B. G.; Yagupolskii, Y. L. A new method for the synthesis of trifluoromethylating agents—Diaryltrifluoromethylsulfonium salts J. Fluorine Chem. 2008, 129, 131–136. doi: 10.1016/j.jfluchem.2007.10.001
- (a) Horváth, I. T., Rábai, J. Facile Catalyst Separation without Water: Fluorous Biphase Hydroformylation of Olefins. Science 1994, 266, 72-75; DOI:10.1126/science.266.5182.72 (b) Handbook of Fluorous Chemistry, Gladysz, J.A.; Curran, D. P.; Horváth, I. T., Eds.; Wiley/VCH: Weinheim, 2004; DOI: 10.1002/3527603905 (c) Fluorous Chemistry, Volume Editor: Horváth, I.T.; Topics in Currant Chemistry, Springer, Vol. 308, 2012; Heidelberg. DOI 10.1007/978-3-642-25234-1.
- (a) Feiring, A. E. Perfluoroalkylation of Thiols . Evidence for a Radical Chain Process J. Fluorine Chem. 1984, 24, 191–203. doi:10.1016/S0022-1139(00)85203-3; (b) Feiring, A. E.; Wonchoba, E. R.; Arthur, S. D. J. Polym. Sci. Polym. Chem. Fluorinated Poly (ether Sulfone)s1990, 28, 2809–2819. doi:10.1002/pola.1990.080281018; (c) Boiko, V. N. Aromatic and heterocyclic perfluoroalkyl sulfides. Methods of preparation Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88
- Harsányi, A.; Dorkó, É.; Csapó, Á.; Bakó, T.; Peltz, Cs.; Rábai, J. Convenient synthesis and isolation of trifluoromethylthio-substituted building blocks. J. Fluorine Chem. 2011, 132, 1241–1246. doi:10.1016/j.jfluchem.2011.07.008
- Unpublished results of PhD student Mr. Bálint Menczinger, Institute of Chemistry, Eötvös Loránd University, Budapest.
- Cf. Harsányi, A. Synthesis and Characterization of Aryl Perfluoroalkyl Sulfides, B.Sc. Thesis, 2010, Institute of Chemistry, Eötvös Loránd University, Budapest.
- Sviridova, A. V.; Laba, V. I.; Vasil’ev, S. V.; Litvinov, V. P. Efficient method for the synthesis of [2-(alkylarylthio)ethyl]pyridines. Russ. Chem. Bull. Int. Ed. 2001, 50, 563-565.
Recommended for publication by Prof. József Rábai
Fluorine Notes, 2017, 114, 1-2
