Received: October 2017
DOI 10.17677/fn20714807.2017.06.01
Fluorine Notes, 2017, 115, 1-2
Addition of thiols to gem-difluoroalkenes under photoactivation conditions
Salavat S. Ashirbaev,1,2 Vitalij V. Levin,1 Marina I. Struchkova,1 Alexander D. Dilman*,1
1N. D. Zelinsky Institute of Organic Chemistry, 119991 Moscow, Leninsky prosp. 47, Russian Federation
dilman@ioc.ac.ru
2 Moscow State University, Department of Chemistry, 119991, Moscow, Leninskie Gory 1-3, Russian Federation
Absrtact gem-Difluoroalkenes interact with thiols in the presence of catalytic amounts of disulfides affording α,α-difluorosubstituted sulfides. The reaction proceeds at room temperature upon irradiation with 400 nm light.
Keywords gem-difluoroalkenes, thiols, photocatalysis
gem-Difluoroalkenes constitute an interesting class of organofluorine compounds. They can be used in medicinal chemistry, and several biologically active molecules bearing the difluorovinyl fragment have been described [1]. On the other hand, difluoroalkenes can serve as precursors in the synthesis of monofluoro- [2], difluoro- [3], and trifluorosubstituted compounds [4]. The difluoroolefines can be readily obtained from carbonyl compounds [5], diazoalkanes [6], and by other methods [2,7].
Recently, an addition reaction of thiols to difluoroalkenes mediated by base 1,1,3,3-tetramethylguanidine was described [8]. The reaction involves nucleophilic attack of the thiolate anion at the electrondefficient duble bond with subsequent protonation and proceeds at elevated temperature. Previously, it was also reported that the addition of thiols to difluoroalkenes can be performed in the presence of benozyl peroxide, though the heating was required, and the products were obtained in moderate yields [9]. In the present work, we report that the addition reaction can be efficiently performed under mild neutral conditions if the process is mediated by light.
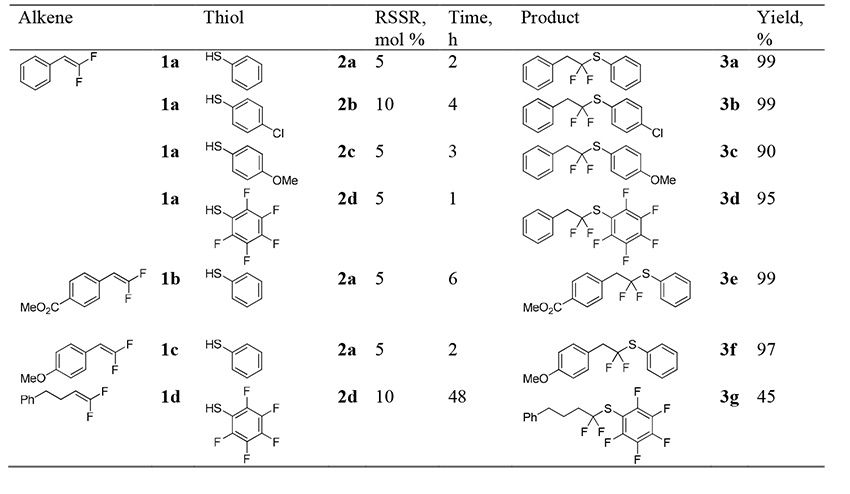
The reaction of difluoroalkenes 1 with thiols 2 was performed in dichloromethane at room temperature upon irradiation with a strip of light emitting diodes with the wavelength of 400 nm (Table 1). Corresponding disulfides were used as catalysts in amount of 5-10 mol %. The reactions with difluoroalkenes bearing aromatic group gave products in more than 90% yields, with the fastest reaction observed with pentafluorothiophenol 2d. At the same time, the interaction of alkyl-substituted substrate 1d with pentafluorothiophenol was very slow, and even after two days, the yield of product 3g was only 45%.
Table 1. Reaction of difluoroalkenes with thiols.
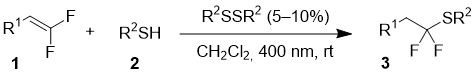
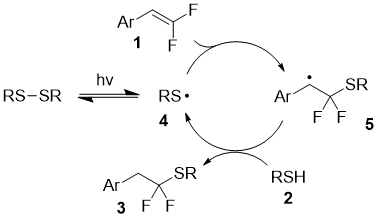
The proposed mechanism involves the generation of S-centered radical 4 upon irradiation of the disulfide. Subsequent addition of the radical at the double bond of the difluoroalkene leads to intermediate 5, which abstracts the hydrogen atom from the starting thiol with the formation of the product and regeneration of radical 4.
Scheme 1. Proposed reaction mechanism.
In summary, we demonstrated that addition of thiols to gem-difluoroalkenes can be effected upon light irradiation. Mild conditions and simplicity of the reaction set-up make this method attractive for the synthesis of α,α-difluorosubstituted sulfides.
Experimental part
Synthesis of disulfides 3 (general procedure). Under argon atmosphere, a solution of difluoroalkene 1 (0.75 mmol), thiol 2 (0.94 mmol) and disulfide (for 3a,c-f, 0.038 mmol; for 3b,g, 0.075 mmol) in dichloromethane (2 mL) was stirred upon irradiation with light emitting diodes (2x smd 3528, Arlight RT 2-5000 12V UV400 2X), with cooling of the reaction vessel with room temperature water. The reaction time is shown in Table 1. Then, the reaction mixture was concentrated under vacuum, and the residue was purified by column chromatography on silica gel.
[(1,1-Difluoro-2-phenylethyl)thio]benzene (3a) [10].
Yield 190 mg (99%), colorless oil, Rf 0.30 (EtOAc/hexane, 1/30). 1H NMR (300 MHz, CDCl3) δ: 3.52 (t, 2H, J = 14.8 Hz) 7.36–7.50 (m, 8H), 7.68 (dd, 2H, J = 7.8, 1.4 Hz). 13C NMR (75 MHz, CDCl3) δ: 45.3 (t, J = 24.3 Hz), 127.1 (t, J = 2.2 Hz), 127.8, 128.5, 128.8 (t, J = 280.0 Hz), 129.1, 129.8, 130.6, 132.1 (t, J = 3.3 Hz), 136.2. 19F NMR (282 MHz, CDCl3) δ: –72.5 (t, J = 14.8 Hz).
1-Chloro-4-[(1,1-difluoro-2-phenlethyl)thio]benzene (3b).
Yield 215 mg (99%), white crystals, mp 63–64 °C, Rf 0.40 (EtOAc/hexane, 1/25). 1H NMR (300 MHz, CDCl3) δ: 3.49 (t, 2H, J = 14.8 Hz), 7.30–7.45 (m, 7H), 7.55 (d, 2H, J = 8.5 Hz). 13C NMR (75 MHz, CDCl3) δ: 45.3 (t, J = 24.2 Hz), 125.5 (t, J = 2.1), 128.0, 128.6, 128.6 (t, J = 280.6), 129.4, 130.6, 131.9 (t, J = 3.4 Hz), 136.4, 137.5. 19F NMR (282 MHz, CDCl3) δ: –72.1 (t, J = 14.8 Hz). Calcd for C14H11ClF2S (284.02): C 59.05, H 3.89. Found: C 59.07, H 3.80.
1-[(1,1-Difluoro-2-phenlethyl)thio]-4-methoxybenzene (3c).
Yield 190 mg (90%), colorless oil, Rf 0.39 (EtOAc/hexane, 1/10). 1H NMR (300 MHz, CDCl3) δ: 3.51 (t, 2H, J = 14.9 Hz), 3.85 (s, 3H), 6.97 (d, 2H, J = 8.8 Hz), 7.37–7.47 (m, 5H), 7.61 (d, 2H, J = 8.8 Hz). 13C NMR (75 MHz, CDCl3) δ: 45.0 (t, J = 24.5), 55.3, 114.7, 117.5 (t, J = 2.4), 127.7, 128.5, 128.7 (t, J = 279.3 Hz), 130.6, 132.3 (t, J = 3.2 Hz), 138.2, 161.2. 19F NMR (282 MHz, CDCl3) δ: –73.2 (t, J = 14.9 Hz). HRMS (ESI): calcd for C15H14F2OSNa [M+Na] 303.0626; found 303.0638.
[(1,1-Difluoro-2-phenlethyl)thio]pentafluorobenzene (3d).
Yield 244 mg (95%), white crystals, mp 49–51 °C, Rf 0.36 (EtOAc/hexane, 1/25). 1H NMR (300 MHz, CDCl3) δ: 3.57 (t, 2H, J = 14.7 Hz), 7.38–7.48 (m, 5H). 13C NMR (75 MHz, CDCl3) δ: 45.2 (t, J = 23.2 Hz), 101.2 (tm, J = 21.7 Hz), 128.3, 128.4 (t, J = 285.5 Hz), 128.8, 130.6, 131.1 (t, J = 3.5 Hz), 137.9 (dm, J = 256.2 Hz), 143.5 (dtt, J = 258.7, 13.4, 5.0 Hz), 148.9 (dm, J = 249.6 Hz). 19F NMR (282 MHz, CDCl3) δ: –161.2 (m, 2F), –148.7 (tt, 1F, J = 20.8, 4.5 Hz), –129.9 (m, 2F), –70.1 (tt, 2F, J = 14.7, 5.8 Hz). Calcd for C14H7F7S (340.02): C 49.42, H 2.07. Found: C 49.28, H 2.01.
Methyl 4-[(2,2-difluoro-2-phenlethyl)thio]-benzoate (3e).
Yield 234 mg (99%), white crystals, mp 70–71 °C, Rf 0.32 (EtOAc/hexane, 1/8). 1H NMR (300 MHz, CDCl3) δ: 3.49 (t, 2H, J = 14.8 Hz), 3.93 (s, 3H), 7.34–7.49 (m, 5H), 7.60 (d, 2H, J = 7.4 Hz), 8.04 (d, 2H, J = 8.3 Hz). 13C NMR (75 MHz, CDCl3) δ: 45.0 (t, J = 24.6 Hz), 52.1, 126.7 (t, J = 2.2 Hz), 128.3 (t, J = 280.0 Hz), 129.1, 129.7, 129.9, 130.6, 136.2, 137.2 (t, J = 3.0 Hz), 166.8. 19F NMR (282 MHz, CDCl3) δ: –72.3 (t, J = 14.8 Hz). HRMS (ESI): calcd for C16H14F2O2SNa [M+Na] 331.0575; found 331.0575.
1-[(2,2- Difluoro-2-(phenylthi)ethyl)]-4-methoxybenzene (3f) [5].
Starting thiophenol cannot be removed from the product by column chromatography. For the isolation of compound 3f, the crude product was dissolved in ethanol (5 mL) followed by addition of hydrogen peroxide (25 l of 30% aqueous soultion, 0.3 mmol) and a crystal of iodine, and the mixture was stirred for 30 min. Then, iodine was neutralized by aqueous solution of Na2S2O3, the mixture was extracted with methyl tert-butyl ether (35 mL). The combined organic phases was dried over Na2SO4, concentrated under vaccum, and the residue was purified by chormatography. Yield 204 mg (97%), colorless oil, Rf 0.32 (EtOAc/hexane, 1/10). 1H NMR (300 MHz, CDCl3) δ: 3.45 (t, 2H, J = 14.7 Hz), 3.85 (s, 3H), 6.96 (d, 2H, J = 8.5 Hz), 7.29 (d, 2H, J = 8.5 Hz), 7.38–7.49 (m, 3H), 7.67 (d, 2H, J = 7.0 Hz). 13C NMR (75 MHz, CDCl3) δ: 44.4 (t, J = 24.3 Hz), 55.2, 114.0, 124.1 (t, J = 3.5 Hz), 127.2 (t, J = 2.1 Hz), 129.0 (t, J = 279.6 Hz), 129.1, 129.7, 131.7, 136.2, 159.4. 19F NMR (282 MHz, CDCl3) δ: –72.4 (t, J = 14.7 Hz).
1,1-Difluoro-4-phenylbutylpentafluorophenylsulfide (3g).
Yield 125 mg (45%), colorless oil, Rf 0.35 (EtOAc/hexane, 1/25). 1H NMR (300 MHz, CDCl3) δ: 1.97–2.11 (m, 2H), 2.18–2.37 (m, 2H), 2.77 (t, 2H, J = 7.5 Hz), 7.20–7.31 (m, 6H), 7.31–7.41(m, 4H). 13C NMR (75 MHz, CDCl3) δ: 24.8 (t, J = 3.3 Hz), 35.0, 38.1 (t, J = 22.4 Hz), 101.3 (tm, J = 18.8 Hz), 126.4, 128.5, 128.7, 129.5 (t, J = 283.5 Hz), 137.9 (dm, J = 256.5 Hz), 140.9, 143.5 (dtt, J = 258.7, 13.7, 5.0 Hz), 148.9 (dm, J = 250.4 Hz). 19F NMR (282 MHz, CDCl3) δ: –161.1 (m, 2F), –148.7 (tt, 1F, J = 21.2, 4.2 Hz), –120.0 (m, 2F), –70.9 (tt, 2F, J = 14.6, 6.3 Hz). MS (EI), m/z: 368.08 [M+], 198.94, 169.08, 149.03, 129.02, 127.00, 105.00, 90.99.
This work was supported by the grants MK-6724.2016.3 and RFBR 16-29-10661.
References
- (a) Magueur, G.; Crousse, B.; Ourevitch, M.; Bonnet-Delpon, D.; Begue, J.-P. J. Fluorine Chem. 2006, 127, 637–642. (b) Leriche, C.; He, X.; Chang, C. T.; Liu, H. J. Am. Chem. Soc. 2003, 125, 6348–6349. (c) Pitterna, T.; Böger, M.; Maienfisch, P. Chimia 2004, 58, 108–116.
- Zhang, X.; Cao, S. Tetrahedron Lett. 2017, 58, 375–392.
- (a) McAlpine, I.; Tran-Dube, M.; Wang, F.; Scales, S.; Matthews, J.; Collins, M. R.; Nair, S. K.; Nguyen, M.; Bian, J.; Alsina, L. M.; Sun, J.; Zhong, J.; Warmus, J. S.; O'Neill, B. T. J. Org. Chem. 2015, 80, 7266–7274. (b) Sasada, Y.; Shimada, T.; Ushioda, M.; Matsui, S. Liq. Cryst. 2007, 34, 569–576. (c) Lin, S.-T.; Chen, L.-C.; Lee, C.-J. J. Chem. Res. 2004, 2004, 353–355. (d) Saito, A.; Okada, M.; Nakamura, Y.; Kitagawa, O.; Horikawa, H.; Taguchi, T. J. Fluorine Chem. 2003, 123, 75–80. (e) Lee, C.-C.; Lin, S.-T. Synthesis 2000, 496–498.
- (a) Gao, B.; Zhao, Y.; Ni, C.; Hu, J. Org. Lett. 2013, 16, 102–105. (b) Qiao, Y.; Si, T.; Yang, M.-H.; Altman, R. A. J. Org. Chem. 2014, 79, 7122–7131. (c) Tian, P.; Wang, C.-Q.; Cai, S.-H.; Song, S.; Ye, L.; Feng, C.; Loh, T.-P. J. Am. Chem. Soc. 2016, 138, 15869–15872. (d) Tang, H.-J.; Lin, L.-Z.; Feng, C.; Loh, T.-P. Angew. Chem. Int. Ed. 2017, 56, 9872–9876.
- (a) Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513–7515. (b) Burton, D. J.; Yang, Z.-Y.; Qiu, W. Chem. Rev. 1996, 96, 1641–1716.
- (a) Hu, M.; Ni, C.; Li, L.; Han, Y.; Hu, J. J. Am. Chem. Soc. 2015, 137, 14496–14501. (b) Zheng, J.; Lin, J.-H.; Yu, L.-Y.; Wei, Y.; Zheng, X.; Xiao, J.-C. Org. Lett. 2015, 17, 6150–6153. (c) Zhang, Z.; Yu, W.; Wu, C.; Wang, C.; Zhang, Y.; Wang, J. Angew. Chem. Int. Ed. 2016, 55, 273–277.
- Ichikawa, J. J. Fluorine Chem. 2000, 105, 257–263.
- Orsi, D. L.; Easley, B. J.; Lick, A. M.; Altman, R. A. Org. Lett. 2017, 19, 1570–1573.
- Suda, M. Tetrahedron Lett. 1981, 22, 2395–2396.
- Ashirbaev, S. S.; Levin, V. V.; Struchkova, M. I.; Dilman, A. D. J. Fluorine Chem. 2016, 191, 143–148.
Recommended for publication by Prof. Alexander D. Dilman
Fluorine Notes, 2017, 115, 1-2
