Received: November 2017
DOI 10.17677/fn20714807.2017.06.04
Fluorine Notes, 2017, 115, 7-8
A comparative estimate of the electron-withdrawing effect of polyfluorinated substituents on the polarization of the O-NO2 bond in nitro esters of perfluorocarboxylic acids
Victor P. Zelenov1, Alexander N. Subbotin1, Ivan A. Trojan2
1N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences,
47 Leninsky prosp., 119991, Moscow, Russian Federation
e-mail: zelenov@ioc.ac.ru
2FSRC “Crystallography and Photonics”, Russian Academy of Sciences,
59 Leninsky Prospekt, 119333, Moscow, Russian Federation.
Abstract: Spectral (Raman and 14N NMR spectroscopy) and quantum-chemical methods were used for comparative estimation of the electron-withdrawing effect of a fluorine-containing substituent on the polarization of the nitro ester bond in nitro esters of perfluorocarboxylic acids. It has been shown that the electron-withdrawing effect of the trifluoromethyl group is somewhat stronger than that of the fluorine atom.
Keywords: nitrate ester of perfluorocarboxylic acid, electron-seeking group, bond polarization
It is known that the nitronium cation, NO2+, is the most efficient nitrating species[1]. Covalent nitro esters of carboxylic acids also react as nitrating agents [2,3]. Obviously, polarization of the O2N-O bond in a nitro ester by electron-withdrawing groups (EWG) located at the β carbon atom results in a redistribution of charges on the oxygen and nitrogen atoms (Scheme 1) and should favor the electrophilic replacement of a proton by a nitro group. An increase in the number of EWGs in a nitro ester molecule elongates the O2N-O bond. Charge separation to give a nitronium cation, i.e., ionization, may be considered as the limiting case of such polarization. Within the scope of ongoing studies on the structure-property relationship in the series of O-nitro compounds, we have previously obtained trifluoroacetyl nitrate (TFAN) and studied its behavior in solutions of acids [3,4,5,6]. In this work, the structure and properties of 2,2,3,3,3-pentafluoropropionyl nitrate (PFPN) by spectral and quantum-chemical methods was investigated.

Scheme 1
One of the principal goals of the study was to find out how the replacement of a fluorine atom in a trifluoroacetyl nitrate molecule by a trifluoromethyl moiety (TFAN → PFPN) alters the properties of the perfluorocarbonyl acid nitro ester. It is convenient to perform this comparison by quantum-chemical calculations and by physicochemical methods, e.g., by estimating the changes in the frequencies of the nitro group symmetric vibrations, νsNO2, in the Raman spectra and the change in δ for the nitrogen signal in the 14N NMR spectra (see Ref. [3]).
It should be noted that studies of the electron-withdrawing effect of CF3 and C2F5 groups in the series of compounds (CH3)3SnCF by the Mössbauer effect studing [7] as well as by the methods of vibrational spectroscopy of the corresponding perfluoro acids [8] were previously carried out, but led to diametrically opposite conclusions.
The NO2+ cation, including a monodently bound to an oxygen of the anion, is a nearly linear structure and the νsNO2 frequency in the Raman spectra of nitronium salts is a characteristic frequency [9]. However, the symmetric vibration of the nitro moiety in covalent nitro esters is not characteristic since it involves the entire molecule and is more strongly affected by conjugation with the carbonyl group. We have shown previously that the experimental Raman spectrum of TFAN is in good agreement with the calculated spectrum of the syn-conformer of the nitro ester whose nitro group is turned by 90° with respect to the C-C(O)-O plane [3]. In the current study we assumed that, if the syn-conformation of the molecule is preserved, replacement of a fluorine atom in the trifluoromethyl group of TFAN by the heavier CF3 substituent through three bonds would affect the symmetric NO2 vibration less strongly in comparison with the additive electron-withdrawing effect of the C2F5- substituent. Thus, the frequency of the symmetric vibration of the nitro group (νsNO2) in the Raman spectra of compounds could serve as a one of the parameters for estimating the electron-withdrawing effect of a substituent.
The electron-withdrawing effect of substituents on the shift of the ONO2 moiety signal (ΔδN) in the 14N NMR spectra of nitro esters (if the solvent is the same) was discovered by us previously [3] and was used in this study as a second parameter for comparison.
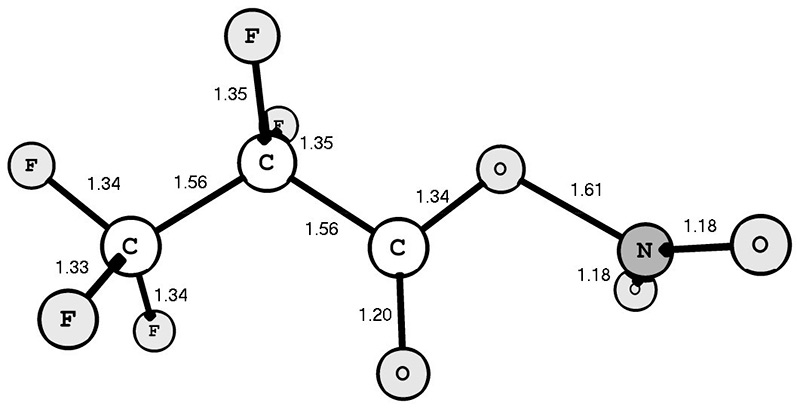
According to the calculation data obtained, the syn-conformation of PFPN (Fig. 1) is 6.17 kcal/mol more beneficial than the anti-conformation. It is important to note that the O-NO2 bond of the PFPN syn-conformer (1.61 Å) is much longer than the similar bond of the TFAN syn-conformer (1.56 Å [3]).
Fig. 1. Structure of the syn-conformer of 2,2,3,3,3-pentafluoropropionyl nitrate. The bond lengths are specified in Å.
To perform an experimental synthesis of pentafluoropropionyl nitrate, we had to bear in mind the specific features of the synthesis of nitro esters containing electron-withdrawing substituents. In fact, the reaction of N2O5 with trifluoroacetic anhydride takes a long time (up to 7 days), unlike its reaction with acetic anhydride (0.5 h) [3]. The synthesis of TFAN is an equilibrium reaction shifted to the right. An equimolar mixture of trifluoroacetic anhydride and nitrogen pentoxide gave TFAN containing less than 10 mol. % of the starting trifluoroacetic anhydride. In the gas phase, TFAN is liable to homolytic decomposition into the anhydrides, their content may reach 15 molar % [3] (Scheme 2). Apparently this is the reason why concentrated TFAN readily explodes upon mechanical impact, even if cooled with ice. However, the synthesis times of the nitro esters are nearly the same in reactions of HNO3 with both acetic and trifluoroacetic anhydride (~10 min at 10 °С) [3].
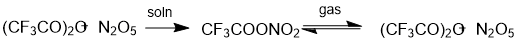
Scheme 2
Table 1. Spectral characteristics of nitro esters
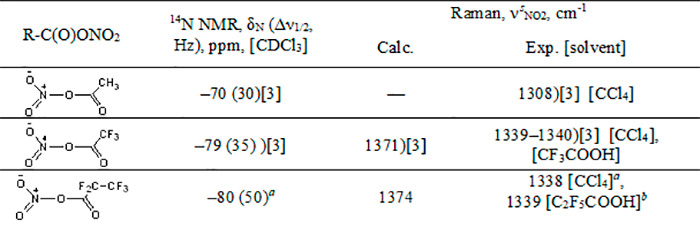
а PFPN was obtained from an equimolar mixture of pentafluoropropionic anhydride and N2O5 (5 °С, 5 days).
а PFPN was obtained from an equimolar mixture of pentafluoropropionic anhydride and HNO3 (0–5 °С, 2 h).
PFPN was obtained by two alternative methods, namely, by reactions of 2,2,3,3,3-pentafluoropropionic anhydride with N2O5 or with HNO3 (Scheme 3). The spectral characteristics of the resulting solutions are presented in Table 1 and in Figs. 2,3. The Raman spectra of 2,2,3,3,3-pentafluoropropionic acid and its anhydride are shown in Figs. 4 and 5.
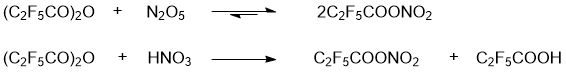
Scheme 3
The experimental values of νsNO2 Raman spectral frequencies of TFAN and PFPN are similar, their difference is within the accuracy of the device. However, quantum-chemical calculations of the PFPN Raman spectrum in the gas phase give somewhat higher νsNO2 frequencies (by ~3 cm-1) in comparison with the calculated spectrum of TFAN, while in the 14N NMR spectra of the compounds in question the signal of PFPN nitrogen is shifted upfield by ~1 ppm in comparison with TFAN (Table 1). These facts, along with the increased length of the nitro ester bond in PFPN (1.61 Å), allow us to believe that the electron-withdrawing effect of the trifluoromethyl substituent is somewhat stronger than that of the fluorine atom. It should be noted that the electron-withdrawing effect of the CF3 substituent on the nitro ester bond is weakened since it is effected through three bonds.
The electron-withdrawing effects of F and CF3 substituents can also be compared by comparing the characteristic sNO2 frequencies of the corresponding nitronium sulfates and the shifts of the 14N signal of the nitronium cations in these salts. In nitronium salts NO2+[FSO3]- and NO2+[CF3SO3]- these substituents affect the nitronium cation through two bonds, therefore the electron-withdrawing effect of the CF3 substituent in comparison with that of the fluorine atom is more distinct than in nitro esters of perfluorocarboxylic acids. The νsNO2 line of NO2+[CF3SO3]- at 1410 cm-1 is appreciably shifted to short wavelength field with respect to the similar frequency of NO2+[FSO3]- (1404 cm-1).7 In the 14N NMR spectra of these salts, the difference in the shifts, ΔδN, reaches 2 ppm (in sulfolane, δN for NO2+[FSO3]- –131, for NO2+[CF3SO3]- –133).7
Thus, we performed a comparative estimate of the effect of polyfluorinated substituents on the degree of polarization of nitro ester bonds in nitro esters of perfluorocarboxylic acids. It has been shown that 2,2,3,3,3-pentafluoropropionyl nitrate exists in the syn-conformation. Furthermore, the electron-withdrawing effect of the trifluoromethyl substituent on the nitro ester moiety through three bonds is similar to, and slightly higher than, the electron-withdrawing effect of the fluorine atom.
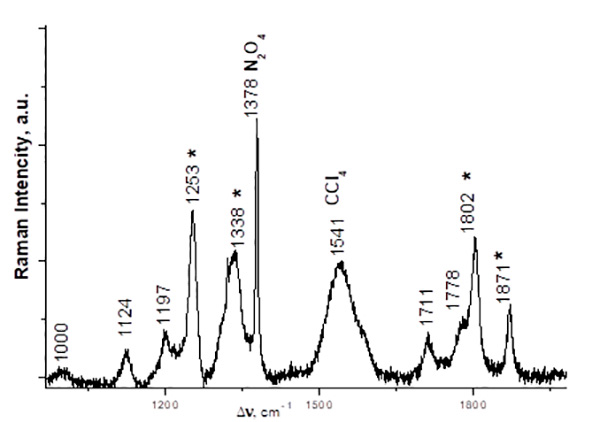
Fig. 2. Raman spectrum of a CCl4 solution of C2F5COONO2 obtained from an equimolar mixture of (C2F5CO)2O and N2O5, 5 °С, 5 days (in the 900–1900 cm-1 region). The frequencies corresponding to C2F5COONO2 spectrum are marked with asterisks. N2O4 was formed due to spontaneous decomposition of N2O5.
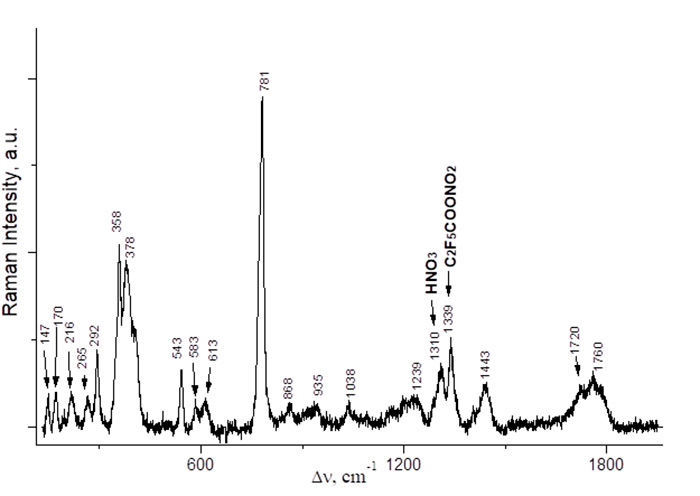
Fig. 3. Raman spectrum of a mixture of C2F5COONO2 and C2F5COOH (≈ 1 : 1) obtained from an equimolar mixture of (C2F5CO)2O and HNO3 (0–5 °C, 2 h).
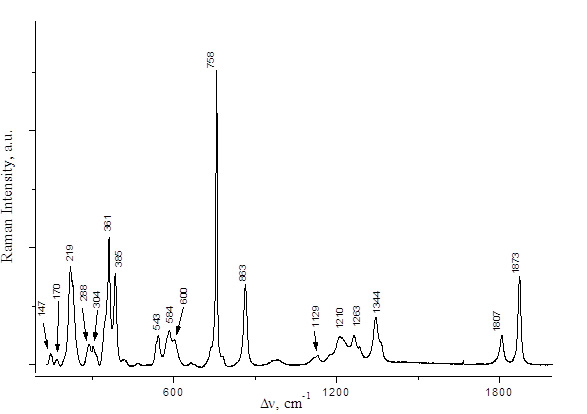
Fig. 4. Raman spectrum of (C2F5CO)2O.
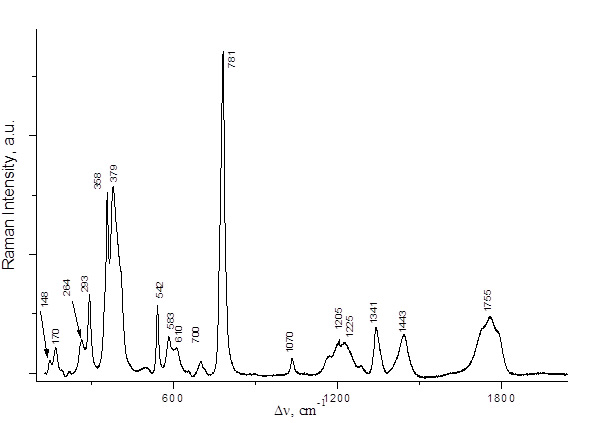
Fig. 5. Raman spectrum of C2F5COOH.
This study was financially supported by the Russian Foundation for Basic Research, Project no. 16-03-00713_a. We are grateful to Closed Joint Stock Company “PiM-Invest” for the sample of 2,2,3,3,3-pentafluoropropionic anhydride and to M. I. Struchkova (Ph.D., Chemistry) who performed the 14N NMR spectroscopic measurements.
Experimental
Nitric acid (d20 = 1.5 g·cm-3) was preliminarily purified by distillation in vacuo, the colourless fraction was used. Dinitrogen pentoxide was prepared by the known procedure from HNO3 and P2O5.3 Raman spectra were recorded with an Acton Research spectrometer with 500 mm focus distance, on a 1200 lines/mm grid. A CCD from Princeton Instruments cooled with liquid nitrogen was used as the light-sensitive detector for the optical signal. The spectra were measured on the 641.7 nm line of a krypton laser with up to 100 mW power, in the 180° Raman line scattering configuration (back scattering). The spectral resolution was 1.5 cm-1. The optical path of the instrument was designed by the confocal microscope scheme with a spatial resolution of 2 μm. To record the spectra, individual compounds were placed into glass ampoules and tightly sealed; reaction mixtures were prepared by mixing the components directly in ampoules. Ampoules with solutions containing C2F5COONO2 were kept in ice until the Raman spectra were recorded; the irradiation power used to record these spectra did not exceed 10 mW. The 14N NMR spectrum (21.69 MHz) was recorded on a Bruker AM300 instrument using CH3NO2 (10 mg) as the inner standard; the upfield shifts are negative. The quantum-chemical calculations were performed using the Gaussian 09 Revision D.01 DFT B3LYP/6-311++g(d,p) software complex [10].
References
- G. A. Olah, G. K. Surya Prakash, Á. Molnár and J. Sommer, Heterocations in Superacid Systems, in Superacid Chemistry, Second Ed., John Wiley & Sons, Hoboken, NJ, USA, 2009, ch. 4.
- J. Hoare, R. Duddu, R. Damavarapu, Org. Process Res. Dev., 2016, 20 (3), 683–686. DOI: 10.1021/acs.oprd.6b00010
- V. P. Zelenov, S. S. Bukalov, L. A. Leites, R. R. Aysin, A. N. Subbotin, М. I. Struchkova, I. V. Fedyanin, Mendeleev Commun., 2017, 27, 31–34; doi: 10.1016/j.mencom.2017.01.009.
- В. П. Зеленов, С. С. Букалов, М. И. Стручкова, А. Н. Субботин, Fluorine Notes, 2016, 4 (107), 179 (Р-88). http://notes.fluorine1.ru/public/2016/4_2016/conference/Program_Thesis_RUS_opt.pdf
- В. П. Зеленов, С. С. Букалов, А. О. Дмитриенко, И. В. Федянин, FluorineNotes, 2016, 4 (107), 90 (O-46). http://notes.fluorine1.ru/public/2016/4_2016/conference/Program_Thesis_RUS_opt.pdf
- V. P. Zelenov, S. S. Bukalov, A. N. Subbotin, Mendeleev Commun., 2017, 27, 355–356; doi: 10.1016/j.mencom.2017.07.011.
- W. R. Cullen, J. R. Sams M. C. Waldman, Inorg. Chem.. 1970, 9, 1682–1686.
- G. A. Crowder, J. Fluorine Chem., 1971/72, 1, 385–389.
- V. P. Zelenov, S.S. Bukalov, I. S. Bushmarinov, L.A. Leites, М. I. Struchkova, A. O. Dmitrienko, V. А. Tartakovsky, Syntheses of nitronium salts. 1. A new strategy towards solid nitronium monosulfates, ChemistrySelect, 2017 (впечати).
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2013.
Recommended for publication by Prof. Sergey Igumnov
Fluorine Notes, 2017, 115, 7-8
