Received: July 2018
DOI 10.17677/fn20714807.2018.04.02
Fluorine Notes, 2018, 119, 3-4
Synthesis and structure of 4,6-bis(difluoromethyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile
Buryi D. S.1, Aksenov N. A.2, Dotsenko V. V.1,2
1Kuban State University, 149 Stavropolskaya str., 350040 Krasnodar , Russian Federation
2North-Caucasus Federal University, 1 Pushkina str., 355009 Stavropol, Russian Federation
e-mail: victor_dotsenko_@mail.ru
Abstract: The Guareschi-Thorpe reaction of 1,1,5,5-tetrafluoroacetylacetone with cyanothioacetamide leads to the formation of 4,6-bis(difluoromethyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile. The structure of the product was studied by means of NMR and X-Ray diffraction methods.
Keywords: 1,1,5,5-tetrafluoroacetylacetone, cyanothioacetamide, Guareschi-Thorpe pyridine synthesis, X-Ray analysis.
The fluorine-containing azaheterocycles have proven to be a class of compounds of great practical interest. Thus, pyridines and their condensed analogs bearing a fluorine-containing side chain were recognized as good microbicides [1], strong analgesics [2], antiviral agents [3], non-receptor tyrosine kinase c-Src inhibitors for cancer therapy [4], etc. Also, fluorine-containing pyridines were used as precursors for thieno[2,3-b]pyridine-based azo dyes useful for dyeing of polyester fibers [5]. In general, fluorine-containing heterocycles can be easily prepared either by reaction of fluorine-containing 1,3-dicarbonyls with dinucleophilic agents [6], or through substitution/condensation reactions with polyfluoroalkenes [7]. One of the most convenient and common approaches towards fluoroalkyl-substituted pyridines is based on the Guareschi-Thorpe cyclization of fluorine-containing 1,3-dicarbonyls (or their masked analogs) with active methylene amides, thio- and selenoamides [1,8-13].
Scheme 1
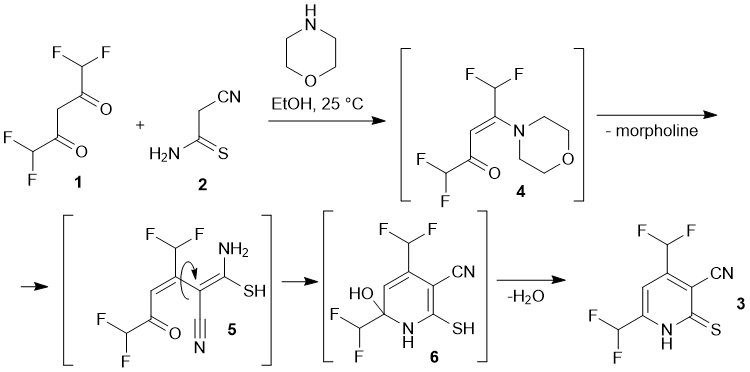
However, the scope of the reaction is still limited by the use of diketones and ketoesters bearing trifluoromethyl substituents mostly. These considerations prompted us to conduct additional studies with other fluorinated diketones to extend the scope of the fluoroalkyl-substituted pyridines, which we intend to use as precursors for the synthesis of new fluorine-containing azines. In the present paper we describe the reaction between 1,1,5,5-tetrafluoroacetylacetone 1 with cyanothioacetamide 2. We found that the reaction proceeds under mild conditions in the presence of catalytic amounts of a secondary amine to give previously unknown 4,6-bis(difluoromethyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3 in moderate yields (Scheme 1).
Presumably, the reaction proceeds through the formation of non-isolable intermediates 4-6. Pyridine 3 was obtained as big red-orange crystals, insoluble in water, sparingly soluble in cold EtOH (but readily – in hot), and also soluble in acetone, DMF and DMSO. The structure of 3 was studied by means of NMR (1H, 13C DEPTQ, 19F, 1H–13C HSQC, 1H–13C HMBC) and X-ray diffraction method (Fig. 1).
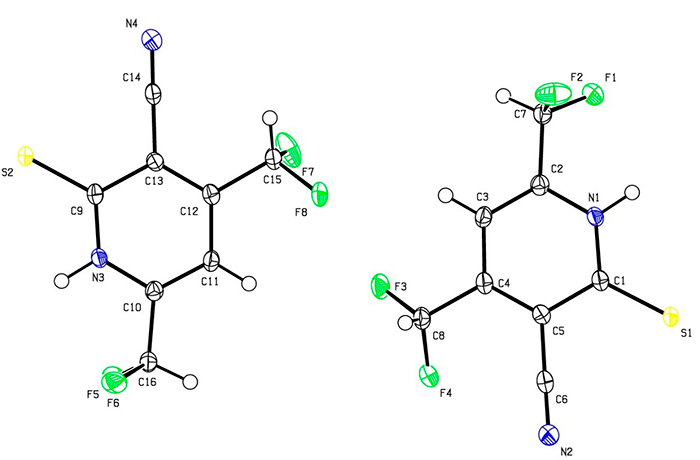
Figure 1. The structure of 4,6-bis(difluoromethyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3 (X-ray data)
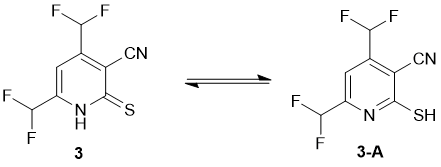
Both X-ray data and IR spectra showed that in a solid state compound 3 exists as the thioxo tautomer. Thus, IR spectrum revealed absorption bands at 3175, 3096 cm-1 (ν N–H) and 1231 cm-1 (due to C=S), along with characteristic stretching vibrations of C≡N group (ν = 2230 cm-1) and very strong bands at 1096 and 1045 cm-1 corresponding to CHF2. However, the NMR spectra of 3 revealed the double set of signals, probably because of tautomeric equilibrium thioxo form 3 ↔ mercapto form 3A in a solution (Scheme 2).
Scheme 2
The NMR 1H spectrum lacked a signal attributable to the NH proton at δ 12.0-14.0 ppm, probably due to H–D exchange. Instead, two signals for H-5 were observed as singlets at δ 7.81 and δ 8.13 ppm, with integral ratio of ~8:1. The signals from СHF2 groups appeared as two pairs of triplets with coupling constants 2JH-F ranging from 52.8 to 53.8 Hz (Fig. 2):
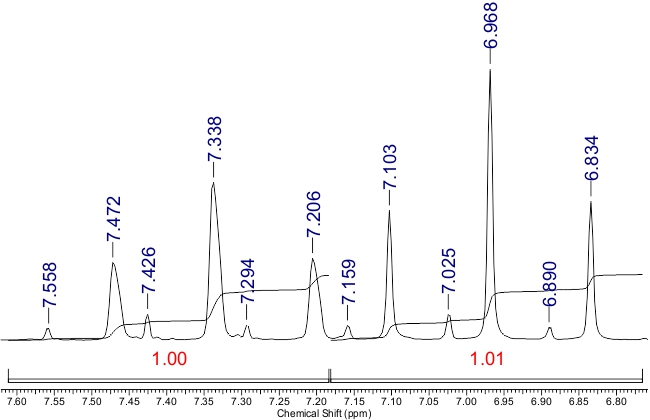
Figure 2. The signals of СHF2 groups in the 1H NMR spectrum of compound 3
The NMR 13С DEPTQ spectrum revealed a signal for CN group at δ 112.6 ppm, partially overlapped signals of both СHF2 groups in the region δ 109-114 ppm with 1JC-F ~ 241 Hz, broadened peaks of C-5 at δ 115.3 ppm (major tautomer) and δ 117.6 ppm (minor tautomer). The signals of C-4 and C-6 appeared downfield as triplets with coupling constants 2JC-F ~ 25 Hz. The most deshielded carbon signal which could be definitely attributed to the signals of a major tautomer, was observed at δ 158.4 ppm. Since the C-2 atom adjacent to mercapto sulfur is typically more shielded compared to the thioxo carbon C=S which is expected to be downfield at δ ~ 175 ppm or more, we suggest that the peak at δ 158.4 ppm can be attributed to C-2 of the major mercapto tautomer 3-A. In the 19F NMR spectra, the signals of each СHF2 of both minor and major tautomers appeared as doublets with coupling constants 2JH-F ranging from 53.2 to 53.8 Hz. In addition, the signals in the NMR spectra were assigned by means of 1H-13C HSQC and 1H-13C HMBC NMR (Fig. 3) experiments. Full set of data of heteronuclear correlations is given in the Table 1. The studies on the reactions of 4,6-bis(difluoromethyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3 are currently underway and will be reported elsewhere.
Table 1. Heteronuclear correlations in the spectra of compound 3
|
1H NMR signals, δ, ppm |
Observed heteronuclear correlations |
|
|
1H-13C HSQC |
1H-13C HMBC |
|
|
6.97 (t, 1Н, C(6)-СHF2) |
111.6 |
115.3; 155.7 |
|
7.34 (t, 1Н, C(4)-СHF2) |
111.5 |
111.3; 147.7 |
|
7.81 (br.s, 1H, H-5) |
115.3 |
111.5; 111.6 |
|
Signals of a minor tautomer |
||
|
7.03 (t, 1Н, C(6)-СHF2) |
111.64 |
154.8 |
|
7.48 (t, 1Н, C(4)-СHF2) |
111.64 |
111.3; 148.1 |
|
8.13 (br.s, 1H, H-5) |
117.6 |
148.1; 154.8 |
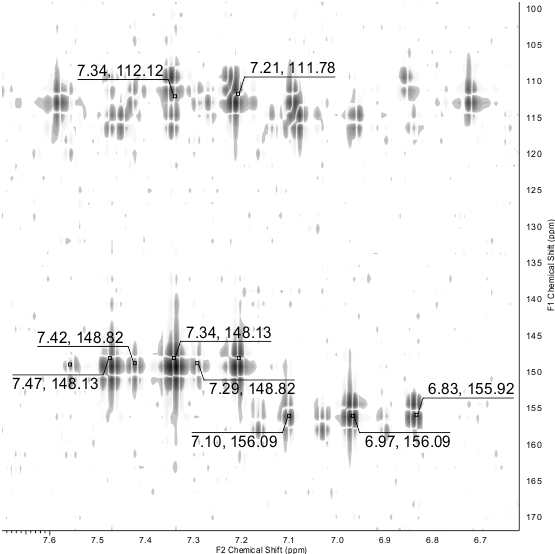
Figure 3. A fragment of 1H-13C HMBC NMR spectrum of compound 3. The cross peaks for СHF2 groups are shown.
Experimental
The NMR 1Н, 13C DEPTQ, 19F spectra were recorded on a Bruker Avance III HD 400 MHz instrument (400.17, 100.63 and 376.5 MHz respectively). Chemical shifts for 1H and 13C are given with respect to the residual peaks of DMSO (δ 2.48 and 39.5 ppm), and for 19F nuclei – with respect to external standard CF3COOH (δ = -76.55 ppm). FTIR spectrum of 3 was recorded on a Bruker Vertex 70 instrument in ATR (attenuated total reflection) mode. Cyanothioacetamide 2 was prepared according to the known procedure [14]. 1,1,5,5-Tetrafluoroacetylacetone (purity 98%) 1 was kindly provided by "P&M-Invest" Ltd. (Moscow, Russia).
4,6-Bis(difluoromethyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3. To a solution of 1,1,5,5-tetrafluoroacetylacetone 1 (3.80 g, 22.08 mmol) in EtOH (15 mL), cyanothioacetamide 2 (2.21 g, 22.07 mmol) and morpholine (0.3 mL) were added under vigorous stirring. A mixture turned red immediately. After thioamide 2 was completely dissolved, a red solution was stirred for another 0.5 h and left to stand overnight. After 24 h, crystalline solid was filtered off, washed with cold EtOH to give 0.98 g (19%) of the desired product 3 as orange-red crystals.
FTIR spectrum, ν, cm-1: 3175, 3096 (N–H), 2230 (CN), 1626, 1587 (C=C), 1352, 1230 (C=S), 1096, 1045 (C–F). Spectrum NMR 1Н, δ, ppm: signals of the major (mercapto) tautomer: 6.97 (t, 1Н, C(6)-СHF2, 2JH-F 53.8 Hz); 7.34 (t, 1Н, C(4)-СHF2, 2JH-F 52.8 Hz); 7.81 (br.s, 1H, H-5). Signals of the minor (thioxo) tautomer: 7.03 (t, 1Н, C(6)-СHF2, 2JH-F 53.8 Hz); 7.48 (t, 1Н, C(4)-СHF2, 2JH-F 52.8 Hz); 8.13 (br.s, 1H, H-5). The ratio of tautomers is ~ 8:1. The signals of NH protons are absent, probably due to H–D exchange. Spectrum NMR 13C DEPTQ, δ, ppm: 111.3 (C-3), 111.5* (t, СHF2, 1JC-F 241.3 Hz), 111.6* (t, СHF2, 1JC-F 241.3 Hz), 112.6 (C≡N), 115.3* (C-5), 147.7 (t, C-4, 2JC-F 25.0 Hz), 155.7 (t, C-6, 2JC-F 25.0 Hz), 158.4 (C-2). Observed signals of a minor tautomer: 111.64* (t, СHF2, 1JC-F 240.6 Hz), 117.6* (C-5), 148.1 (t, C–СHF2, 2JC-F 23.5 Hz), 154.8 (br. peak, C–СHF2). *Signals in opposite phase. Spectrum NMR 19F, δ, ppm: signals of a minor tautomer: -118.76 (d, СHF2, 2JH-F 53.8 Hz); -116.46 (d, СHF2, 2JH-F 53.2 Hz). Signals of a major tautomer: -118.88 (d, СHF2, 2JH-F 53.7 Hz); -116.35 (d, СHF2, 2JH-F 53.2 Hz). Found, %: С, 40,57; Н, 1,79; N, 11,80. C8H4F4N2S (M = 236.19). Calculated, %: C, 40.68; H, 1.71; N, 11.86.
Crystal structure determination of 4,6-bis(difluoromethyl)-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3
Single crystals of compound 3 were prepared by crystallization from EtOH. A suitable crystal was selected and mounted on the glass stick by acrylic glue on a SuperNova Dual Cu at zero, Atlas S2 diffractometer. The crystal was kept at 100.00(11) K during data collection. Using Olex2 [15], the structure was solved with the ShelXT [16] structure solution program using Intrinsic Phasing and refined with the ShelXL [16] refinement package using Least Squares minimisation. Crystal data for compound 3 (C8H4F4N2S, M = 236.19 g/mol): crystals are triclinic, space group P-1 (no. 2), the unit cell parameters at 100 K are as follows: a = 8.4494(6) Å, b = 8.5120(5) Å, c = 12.7799(4) Å, α = 94.167(4)°, β = 97.675(4)°, γ = 107.002(6)°, V = 865.03(9) Å3, Z = 4, T = 100.00(11) K, μ(CuKα) = 3.713 mm-1, Dcalc = 1.814 g/cm3. The intensity of 10072 reflections (7.028° ≤ 2Θ ≤ 152.524°), 3577 independent (Rint = 0.0465, Rsigma = 0.0341) were measured and were used in all calculations. The final divergence factors are as follows: R1 0.0519 (for independent reflections with I > 2σ(I)) and wR2 0.1472 for all reflections. Crystallographic data including atomic coordinate tables, temperature factors, tables of bond lengths and bond angles were deposited at the Cambridge Structural Data Centre (CCDC 1852646).
Acknowledgements
This work was supported by the Ministry of Education and Science of the Russian Federation (projects no. 4.5547.2017/8.9 and 4.1196.2017/4.6).
References
- Kumar G. S., Poornachandra Y., Reddy K. R., Kumar C. G., Narsaiah, B. Synthesis of novel triazolothione, thiadiazole, triazole-functionalized furo/thieno [2, 3-b] pyridine derivatives and their antimicrobial activity. Synthetic Communications, 2017, 47(20), 1864-1873.
- Ryu H., Seo S., Lee J.Y., Ha T.H., Lee S., Jung A., Ann J., Kim S.E., Yoon S., Hong M., Blumberg P.M., Frank-Foltyn R., Bahrenberg G., Schiene K., Stockhausen H., Christoph T., Frormann S., Lee J. Pyridine C-region analogs of 2-(3-fluoro-4-methylsulfonylamino-phenyl) propanamides as potent TRPV1 antagonists. European journal of medicinal chemistry, 2015, 93, 101-108.
- Dai D., Burgeson J. R., Tyavanagimatt S. R., Byrd C. M., Hruby D. E. Thienopyridine derivatives for the treatment and prevention of dengue virus infections U.S. Patent Application No. 13/708,224, 2013.
- Pevet I., Brulé C., Tizot A., Gohier A., Cruzalegui F., Boutin J. A., Goldstein S.Synthesis and pharmacological evaluation of thieno[2,3-b]pyridine derivatives as novel c-Src inhibitors. Bioorganic & medicinal chemistry, 2011, 19(8), 2517-2528.
- Ho Y. W., Yao W. H. Synthesis and properties of heterocyclic monoazo dyes derived from 3-cyano-4-trifluoromethyl-6-substituted-2 (1H)-pyridinethiones. Dyes and pigments, 2006, 70(1), 60-69.
- Burgart Y. V., Saloutin V. I., Chupakhin O. N. Fluorine-containing 2-functionalized 1, 3-dicarbonyl compounds for heterocyclic synthesis. Heterocycles, 2006, 69, 593-620.
- Furin G. G. Synthesis of heterocycles with polyfluoroalkyl substituents from unsaturated compounds containing polyfluoroalkyl groups. Chemistry of Heterocyclic Compounds, 2006, 42(3), 285-319.
- Rodinovskaya L. A., Sharanin Y. A., Litvinov V. P., Shestopalov A. M., Promonenkov V. K., Zolotarev B. M., Mortikov V. Y. Nitrile cyclization reactions. 8. Synthesis and transformation of 6-aryl-4-trifluoromethyl-3-cyano-2 (1H)-pyridinethiones. Zh. Org. Khim. (J. Org.Chem. USSR), 1985, 21(11), 2439-2444 (in Russian).
- Rodinovskaya L. A., Shestopalov A. M., Gromova A. V., Shestopalov A. A. One-pot synthesis of diverse 4-di (tri) fluoromethyl-3-cyanopyridine-2 (1H)-thiones and their utilities in the cascade synthesis of annulated heterocycles. Journal of combinatorial chemistry, 2008, 10(2), 313-322.
- Rateb N. M. Synthesis and reactions of 4-trifluoromethyl-3-cyano pyridine-2 (1H)-thione/one derivatives. Journal of Sulfur Chemistry, 2011, 32(6), 611-622.
- Dyachenko V. D., Tkachev R. P., Dyachenko A. D. Synthesis of 2-thioxo-6-trifluoromethyl-1, 2-dihydropyridine-3-carbonitrile and ethyl 5-cyano-6-thioxo-1, 6-dihydropyridine-2-carboxylate by the SNVin reaction. Russian Journal of General Chemistry, 2009, 79(1), 121-124.
- Eriksson M. C., Zeng X., Xu J., Reeves D. C., Busacca C. A., Farina V., Senanayake C. H. The Guareschi–Thorpe Cyclization Revisited – An Efficient Synthesis of Substituted 2, 6-Dihydroxypyridines and 2, 6-Dichloropyridines. Synlett, 2018, 29(11), 1455-1460.
- Nikishin K. G., Kislyi V. P., Nesterov V. N., Shestopalov A. M., Struchkov Y. T., Semenov V. V. Regioselective synthesis and properties of 3-cyano-6-methyl-4-trifluoromethylpyridine-2 (1H)-thione. Molecular and crystal structure of 3-cyano-2-ethylthio-6-methyl-4-trifluoromethylpyridine. Russian chemical bulletin, 1998, 47(3), 465-468.
- Dotsenko V. V., Krivokolysko S. G., Polovinko V. V., Litvinov V. P. On the regioselectivity of the reaction of cyanothioacetamide with 2-acetylcyclo-hexanone, 2-acetylcyclopentanone, and 2-acetyl-1-(morpholin-4-yl)-1-cycloalkenes. Chemistry of Heterocyclic Compounds, 2012, 48(2), 309-319.
- Dolomanov O. V., Bourhis L. J., Gildea R. J, Howard J. A. K., Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst., 2009, 42, 339-341.
- Sheldrick G. M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallographica Sec. A, 2015, 71(1), 3-8.
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2018, 119, 3-4
