Received: August 2018
DOI 10.17677/fn20714807.2018.05.02
Fluorine Notes, 2018, 120, 3-4
Quantum-chemical recearch of the cationic polymerization of iso-olefin 2-methylbutene-1 in the presence of boron fluoride aqua complex initiation mechanism
V.A. Babkin1, D.S. Andreev1, A.V. Ignatov1, А.I. Rakhimov 2, E.S. Titova2, O.S. Rakhimova 3, V.T. Fomichev2
1Sebryakovsky branch of Volgograd State Technical University, 403343, Volgogradskaya obl.,
Mikhailovka, 2-d microdistrict, Sverdlova street, 2
e-mail: babkin_v.a@mail.ru
2 Volgograd State Technical University, 400131, Russia,
Volgograd, Lenin prospect, 28
e-mail: organic@vstu.ru
3 Volgograd State University, 400062, Volgograd, Universitetskiy prospect, 100
Abstract: The first quantum-chemical research of the initiation mechanism of cationic polymerization of 2-methylbutene-1 in the presence of boron fluoride aqua complex by the ab initio method in 3-311G** basis with the geometry optimization in all parameters using the gradient method when initial particle attacks α- and β-carboxylic monomer’s atoms has been performed. We have established that these reactions are exotermic and have the barrier character. The energy barrier when initial particle attacks iso-olefin α- and β-carboxylic atom is equal to 147 kJ/mol and the heat of reaction is equal to 42 kJ/mol, on α- and β-carboxylic atom – 186 kJ/mol and 32 kJ/mol correspondingly. The reaction initiated according to the Markovnikov’s rule (attack on iso-olefin α- carboxylic atom) on 39 kJ/mol is energetically profitable.
Key words: iso-olefin, 2- methylbutene-1, boron fluoride, initiation mechanism, ab initio method, energy barrier, heat of reaction.
Introduction
Boron fluoride aqua complexes are classical catalysts for cationic polymerization of olefins [1]. Down to recent times, the elementary acts mechanisms (initiation and growth) in the presence of these catalysts were studied only for ethylene, propylene and isobutylene [2]. For other olefins (normal, branched, iso-olefins, styroles and so on) these mechanisms are yet not studied. The isobutylene polymerization is intensively investigated and used in practice, but there is not much information about the other substituted ethylenes polymerization behavior. For instance, the first experimental data about 2-methylbutene-1 polymerization were obtained in 1934 [3]. Ola and others [4] declared about the liquid polymers formation when the given olefin contacts with different complexes such as HF-BF3, DF-BF3, RF-BF3 and others. (where R – isopropyl, tert-butyl, cyclohexyl) at -80°C. Due to the fact that acid strength of these complexes is lower than, for example, HF-BF3 acid strength, the usage of boron fluoride aqua complex as a catalyst can drive up selective ability of polymerization processes and decrease heat effect. That is important to know when developing new technical processes of 2-methylbutene-1 polymerization. However, down to recent times, the elementary acts mechanisms of this olefin and initiation mechanism, as a stage where it is possible to rule the polymerization process, have not been investigated. For these reasons, the aim of this work was investigation of mechanism of 2-methylbutene-1 polymerization in the presence of boron fluoride aqua complex.
Methodological part
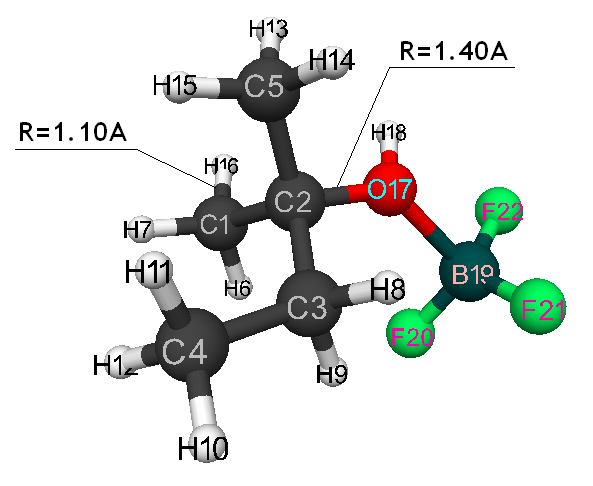
Initiation mechanism of 2-methylbutene-1 cationic polymerization in the presence of BF3∙H2O was studied through building of potential surface of cooperation of catalyst with monomer using the classical quantum-chemical method AB INITIO with geometry optimization in all parameters by gradient method, integrated in Firefly [5], which is partially based on the initial code GAMESS (US) [6]. This method is specially parameterized for the better reproduction of energetic characteristics of molecular systems [7]. The calculations were held in nearing of isolated molecule in gas-phase in molecular model’s frames. The 2-methylbutene-1 initiation mechanism in the presence of boron fluoride aqua complex was held according to the methodology, described in the article [8]. Bond lengths of the studied molecular systems RC2H16 and RC1F17 were chosen as reaction coordinates (Pic. 1). The program MacMolPlt was used for the molecule visual presentation [9].
Results discussion
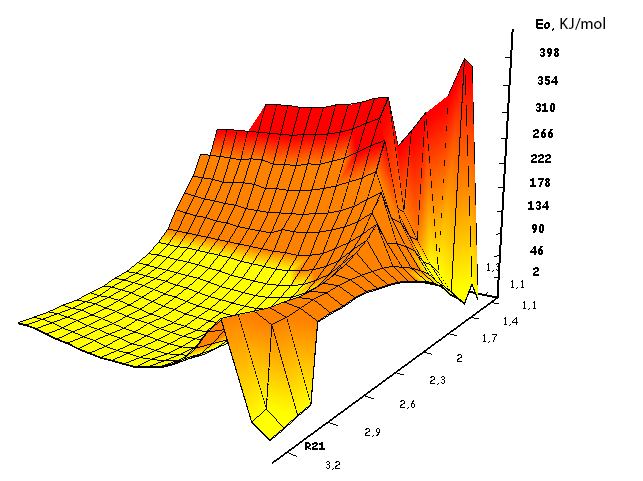
Information about the initiation mechanisms of 2-methylbutene-1 in the presence of BF3∙H2O aqua complex on α- and β-carboxylic atom are shown in the Pic. 1-10. The initial model of iso-olefin 2-methylbutene-1 cooperation with complex catalyst BF3∙H2O when it is attacked by monomer’s initiating particle (proton H16) is shown in the picture 1. The geometrical and electronic structure of the active center when being attacked by monomer of cationic polymerization 2-methylbutene-1 complex initiator BF3∙H2O on iso-olefin’s α-carboxylic atom are shown in the Pic. 2. Cooperation surface of iso-olefin 2-methylbutene-1 with complex initiator BF3∙H2O when initiating particle (proton H16) attacks α-carboxylic monomer’s atom is shown in the Pic. 3. Energy profile of the cooperation reaction of iso-olefin 2-methylbutene-1 with complex catalyst BF3∙H2O when initiating particle (proton H16) attacks monomer’s α-carboxylic atom is illustrated in the Pic. 4. The graph of the charge behavior on atoms of molecular system when cooperation of complex catalyst BF3∙H2O with olefin 2-methylbutene-1 along the way of the reaction RH16C1 (kJ/mol) when initiating particle (proton H16) attacks monomer’s α-carboxylic atom is illustrated in the Pic. 5. The basic model of interaction between iso-olefin 2-methylbutene-1 and complex catalyst BF3∙H2O when being attacked by monomer’s initiating particle (proton H16) is shown in the Pic. 6. In the Pic. 7 – geometrical and electronic structure of the active center when monomer of cationic polymerization 2-methylbutene-1 complex initiator BF3∙H2O attacks iso-olefine’s β-carboxylic atom. In the Pic. 8 one can see the cooperation surface of iso-olefin 2-methylbutene-1 with complex catalyst BF3∙H2O when initiating particle (proton H16) attacks monomer’s β-carboxylic atom. In the Pic. 9 – energetic profile of the cooperation reaction of iso-olefin 2-methylbutene-1 with complex catalyst BF3∙H2O when initiating particle (proton H16) attacks monomer’s β-carboxylic atom. The graph of charges behavior on molecular system atoms at cooperation of complex catalyst BF3∙H2O with olefin 2-methylbutene-1 along the way of the reaction RH16C2 (kJ/mol) when initiating particle (proton H16) attacks monomer’s β-carboxylic atom is shown in the Pic. 10. The process of cooperation of the studied iso-olefin 2-methylbutene-1 with complex catalyst BF3∙H2O when proton attacks monomer’s α-carboxylic atom can be divided into 3 stages (by analogy [10]): the first – coordination stage, the second – stage of monomer’s π-bond breakage and the third – active center’s formation. On the coordination stage (the reaction RH16-C1 and RO17-C2 coordinates vary from 3,1 to 1,4 and from 3,5 to 2,3 nm). On this stage catalyst’s and iso-olefin’s relative orientation takes place, angle of attack of initiating particle on H16+ α-carboxylic atom is calculated. On the stage of π-bond breakage the reaction RH16-C1 and RF17-C2 coordinates vary from 1,4 to 1,3 and from 2,3 to 1,9 nm correspondingly. On the third stage the reaction RH16-C1 and RF17-C2 coordinates vary from 1,3 to 1,1 and from 1,9 to 1,5 nm correspondingly, the formation of active center, that is polarized intermediate –[BF3 ∙ OH]-δ – C(2)+δ [CH+δH2H3CH3C2H5] takes place. On the coordination stage up to 8-9 cooperation step the monomer’s nearing to catalyst is profitable. As it is shown in the Pic. 4, it is characterized by energy minimum of all the molecular system (E0) in this coordination stage. Starting from 10-th to 20-th steps the E0 only grows, from 21-t step it starts fall dramatically, what is characterized by energy barrier of this reaction 147 kJ/mol (Pic. 4). Decrease of general system’s energy is directly connected with the beginning of cooperation between the initiating particle H+δ with iso-olefin α-carboxylic atom and π-bond breakage. On the third cooperation stage the general molecular system energy reaches it’s maximum, that shows the complete active center formation. Alongside this, from the Pic. 4 one can see that this reaction is exothermic and its heating value is 42 kJ/mol.
When α-carboxylic iso-olefin atom 2-methylbutene-1 is attacked by initiating particle, charge on hydrogen atom H16 varies from 0,3 to 0,36. The charge on C1 atom (iso-olefin α-carboxylic atom) varies from -0,28 to -0,3, and its peak is on the π-bond cleavage stage. On the coordination stage the charge on C2 atom also suffers changes. On this stage the charge is negative and varies from -0,153 to -0,35. The charge on the oxygen atom O17 during the reaction process varies from -0,466 to -0,594. Other charges during the reaction process changed unessential. Due to this fact, we studied only charges changes on that atoms, which directly took part in the initiating reaction of 2-methylbutene-1 in the presence of BF3∙H2O.
It is obvious that the cationic polymerization initiating mechanism of the studied monomer in the presence of boron fluoride aqua complex has close interaction lines, what means that during the reaction process breakage of two bonds – RH16-O17 and RC1-C2 takes place at the same time (olefin’s π-bond into σ-transformation) and new bonds - RH16-C1 and RO17-C2 formation.
The process of cooperation of iso-olefin 2-methylbutene-1 with the complex catalyst BF3∙H2O when proton attacks monomer’s β-carboxylic atom, by analogy with the proton attack on α-carboxylic atom can also be divided into three stages: the first – coordination stage, the second – monomer’s π-bond breakage stage and the third – active center formation.
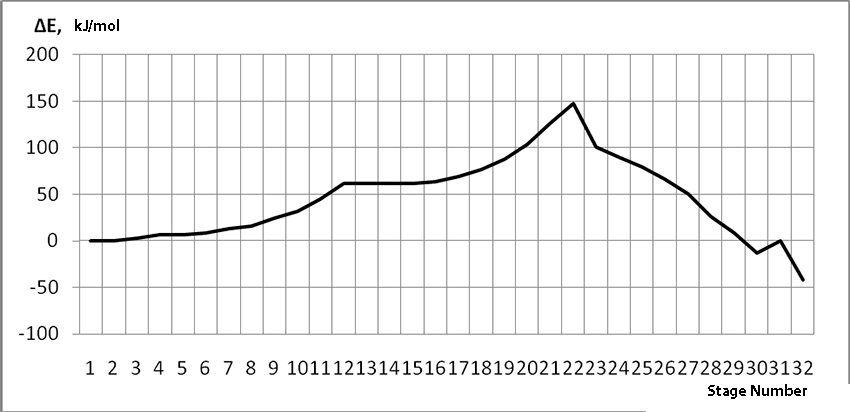
In this case, on the 22 stage, reactions RC1-O17 and RC2-H16 coordinates vary from 3,1 to 1,4 and from 3,5 to 2,2 nm correspondingly. Boron fluoride complex catalyst with iso-olefin relative orientation happens on this stage and initiating particle H16+ on β-carboxylic atom angle of attack is calculated. RC2-H16 reaction coordinates decreases from 1,4 to 1,1, RF17-C2 - from 2,2 to 1,6 nm. On the third stage active center formation takes place. Active center is a polarized intermediate – [BF3 ∙ OH]-δ C(1)+δ [CH+δH2H3CH3C2H5]. On the coordination stage, till 8-9 steps, monomers nearing to catalyst is energetically profitable (Pic. 9). Energy profile of cooperation reaction of iso-olefin 2-methylbutene-1 with complex catalyst BF3 ∙ OH when being attacked by the initiating particle H(16)+δ can be characterized by minimum energy of all molecular system (E0) on this coordination stage. Starting from 10-th stage to 20 stage, the figure E0 only grows up, whether from 21-st stage to 24-th it starts to decrease, what is characterized by energetic barrier of this reaction 186 kJ/mol (Pic. 9). Overage system energy decrease is connected with the beginning of cooperation of initiating particle H(16)+δ with iso-olefin β-carboxylic atom and π-bond breakage. On the third stage of cooperation total energy reaches its maximum, which proves the total active center formation. From the Pic. 9 one can see that the reaction is exothermic and its heating effect is equal to 32 kJ/mol. When iso-olefin 2-methylbuten β-carboxylic atom being attacked by initiating particle, the charge on the hydrogen atom H(16) varies from +0,31 to +0,367. The negative charge on C(1) atom (iso-olefin β-carboxylic atom) on the coordination stage grew from -0,3 to -0,07, and as soon as iso-olefines π-bond breakage starts (20-th stage), it turns out to be positive +0,2, and then decreases +0,124. Charge on C(2) atom grows up in absolute value from -0,153 to -0,35, reaching its maximum in the moment of π-bond breakage, and then it varies -0,3-0,24. Charge on oxygen O(17) atom varies from -0,45 (on the coordination stage) to -0.47, it reaches its maximum (-0,59). Other charges, during the reaction process, changed insignificantly. It is obvious that when β-carboxylic atom is being attacked, the initiating mechanism is the same as when α-carboxylic atom is being attacked. That means that two bonds – RH16-O17 and RC1-C2 breaks (olefin π-bond in transformation) and new bonds – RO17-C1 and RH16-C2 formation take place at the same time
To sum up: quantum-chemical analysis for the initiating mechanism of iso-olefin 2-methylbutene-1 cationic polymerization in the presence of boron fluoride aqua complex, using the ab initio HF/6-311G** method with geometry optimization in all parameter by standard gradient method on each stage of cooperation, when initiating particle attacks monomer’s α- and β-carboxylic atoms, was held for the first time. It was found out that these reactions are exothermic and have barrier nature. Energy barrier in case of iso-olefin α-carboxylic atom is being attacked by initiating particle is – 147 kJ/mol, heat of reaction is -42 kJ/mol, and on β-carboxylic atom – 186 kJ/mol and 32 kJ/mol correspondingly. Initiation reaction according to Markovnikov’s rule (attack on iso-olefin α- carboxylic atom) on 39 kJ/mol is energetically profitable.
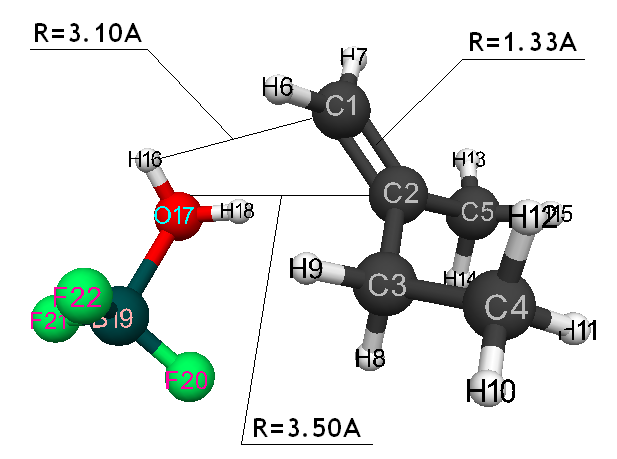
Pic. 1. The initial model of cooperation between 2-methylbutene-1 with BF3 ∙ H2O aqua complex, when monomer’s initiating particle (proton H16) attacks iso-olefin α-carboxylic atom.
Pic. 2. Geometrical and electronic structure of the active center when cationic polymerization monomer 2-methylbutene-1 BF3-H2O aqua complex attacks iso-olefin α-carboxylic atom.
Pic. 3. The surface of cooperation of 2-methylbutene-1 with BF3-H2O aqua complex when the initiating particle (prooton H16) attacks monomer’s α-carboxylic atom.
Pic. 4. The energy profile of cooperation reaction of 2-methylbutene-1 with BF3-H2O aqua complex when initiating particle (proton H16) attacks monomer’s α-carboxylic atom.
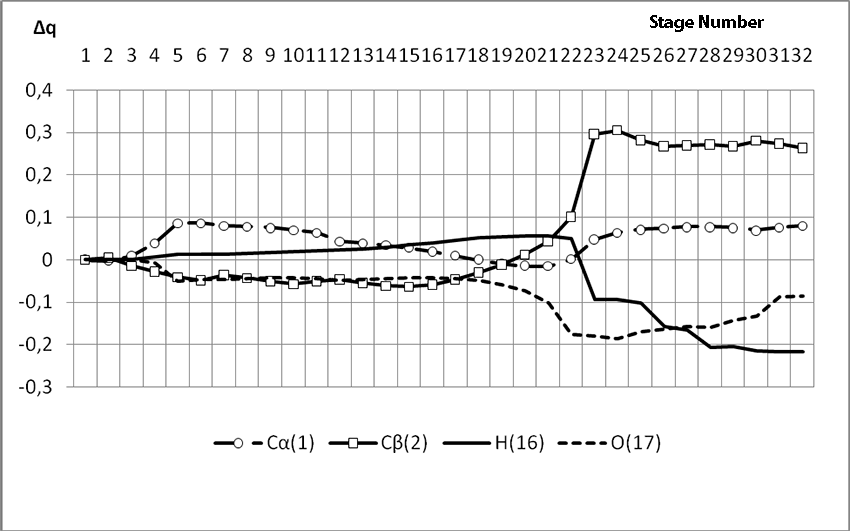
Pic. 5. The graph of charge changes of molecular system atoms in the interaction of BF3-H2O aqua complex with 2-methulbutene-1 along the RH16C1 (kJ/mol) reaction way, when initiating particle (proton H16) attacks monomer’s α-carboxylic atom.
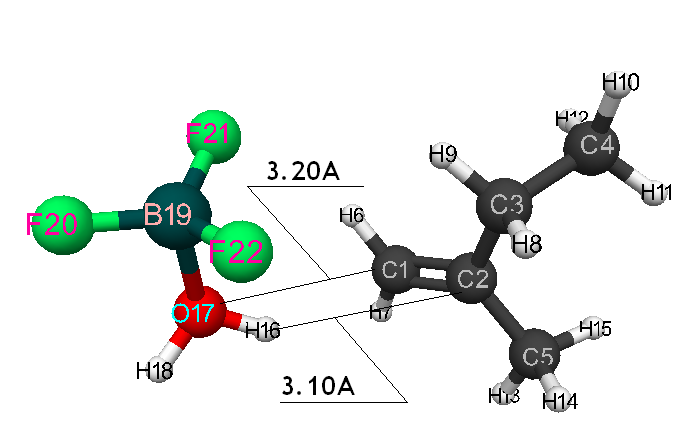
Pic. 6. The initial model of cooperation between 2-methylbutene-1 and BF3-H2O aquacomplex when initiating particle (proton H16) attacks iso-olefin β-carboxylic atom.
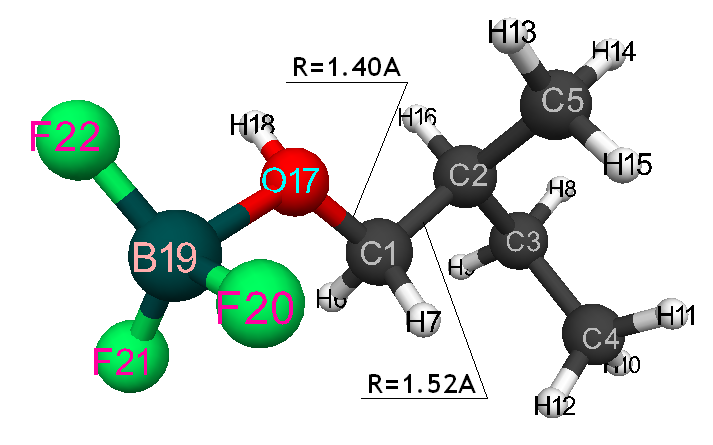
Pic. 7. Geometrical and electronic structure of the active center, when 2-methylbutene-1 BF3-H2O aqua complex attacks iso-olefin β-carboxylic atom.
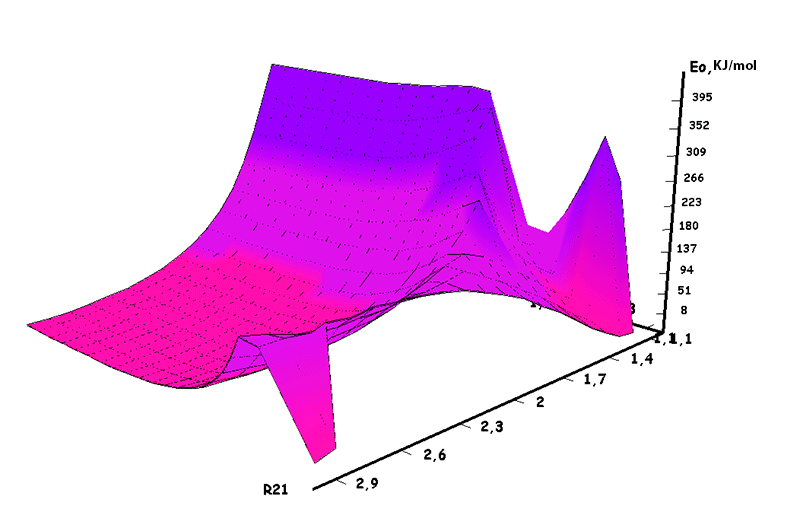
Pic. 8. The surface of cooperation between iso-olefin 2-methylbutene-1 and BF3-H2O aqua complex when initiating particle (proton H16) attacks monomer’s β-carboxylic atom.
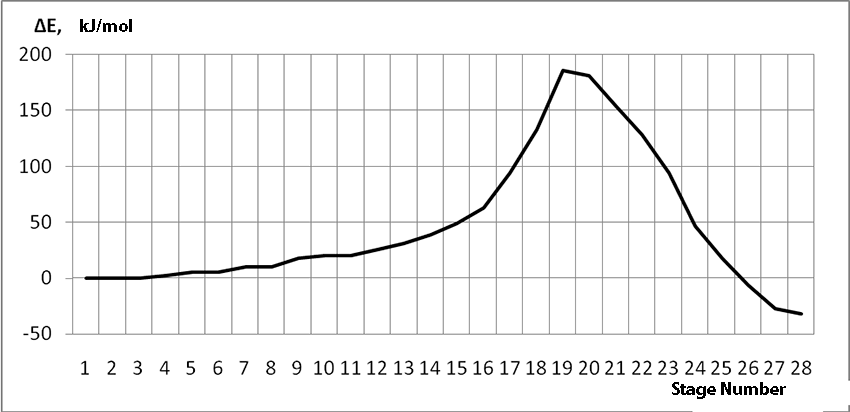
Pic. 9. Energy profile of reaction of cooperation between 2-methylbutene-1 and BF3- H2O aqua complex when initiating particle (proton H16) attacks monomer’s β-carboxylic atom.
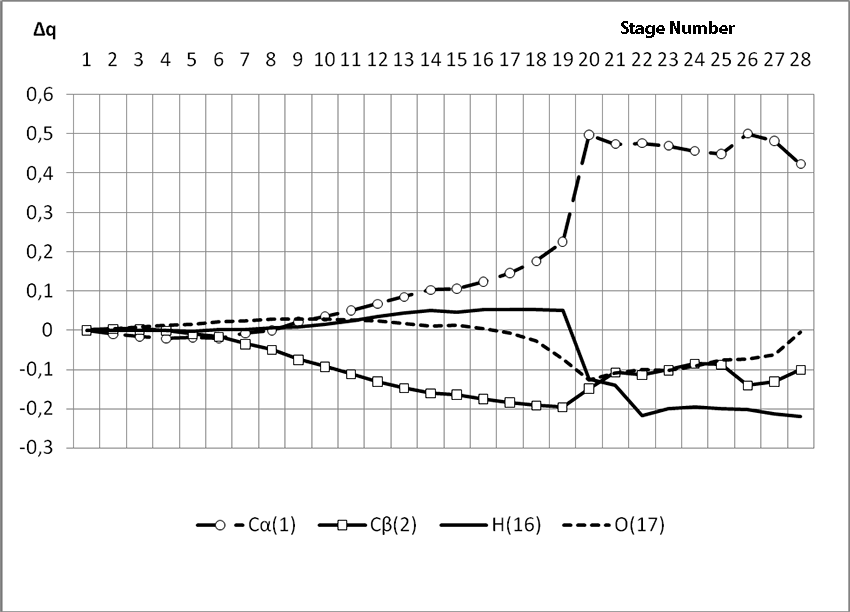
Pic. 10. The Graph of variation of charges on molecular system atoms when BF3-H2O aqua complex cooperates with 2-methylbutene-1 along the reaction RH16C2 (kJ/mol) way when initiating particle (proton H16) attacks monomer’s β-carboxylic atom.
REFERENCES
- Kennedy, J. Cationic Polymerization of Olefins /J. Kennedy. –М., 1978. – Р. 431.
- Babkin, V.А. Quantum-chemical aspects of the cationic polymerization of olefins / V.А. Babkin, G.Е. Zaikov., К.S. Minsker. - Ufa: Academy of Sciences of the Republic of Bashkortostan, 1996. – 188 р.
- Leendertse, J. J. On the behaviour of the pentenes with branched chains and the normal pentenes towards hydrochloric acid and aluminium chloride at low temperatures/ J. J. Leendertse, A. J. Tulleners, H. I. Waterman // Recueil des Travaux Chimiques des PaysBas. – 1934. – Vol. 53. – P. 715–724.
- Olah, G. A., Ionic Polymerization. I. Reaction Mechanism Investigation of the Cationic Polymerization of α-Olefins through Intermediate Carbonium Ion Complexes/ G. A. Olah, H. W. Quinn, S. J. Kuhn // Journal of the American Chemical Society. – 1960. – Vol. 82 (2). – P. 426–430.
- Alex A. Granovsky, Firefly version 8. http://classic.chem.msu.su/gran/firefly/index.html
- General Atomic and Molecular Electronic Structure System/ Schmidt M.W. [and others]// J.Comput.Chem. – 1993. – №14. – Р. 1347-1363.
- Tsirelson, V.G. Quantum chemistry. Molecules, molecular systems and solids / V.G. Tsirelson. - М.:Binom, 2010. - 496 p.
- Andreev, D.S. Quantum-chemical study of the mechanism of iso-olefin initiation o-methylstyrene in the presence of aluminum chloride aqua complex / D.S. Andreev, V.А. Babkin, G.Е. Zaikov // Bulletin of Kazan Technological University. - 2015. - Vol.18 - №1. - P. 28-31.
- Bode, B.M. MacMolPlt: A Graphical User Interface for GAMESS/. B.M. Bode and M.S.. Gordon// J. Molec. Graphics. - 1998. - №16. - Р. 133-138.
Recommended for publication by Prof. A.I. Rakhimov
Fluorine Notes, 2018, 120, 3-4
