Received: October 2018
DOI 10.17677/fn20714807.2018.05.04
Fluorine Notes, 2018, 120, 7-8
Preparation of trifluoromethyl(trimethyl)silane using Swarts reaction
V.E. Boikoab, S.М. Igumnovab
aA.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,Russia, 119991, GSP-1, Moscow, V-334, Vavilova St, 28
b SIA “P&M-Invest” ltd, Russia, 119334 Moscow, Leninsky prospect 47
Abstract: It was described the method of fluorination of Cl-C-Si bond using Swarts reaction on the example of preparation of trimethyl(trifluoromethyl)silane.
Keywords: Swarts reaction, trimethyl(trifluoromethyl)silane, fluorination.
Introduction
Fluorination through the substitution of halogens is a widely used method of preparation of organofluorine compounds both in the laboratory and in the industry. The examples of usage of different inorganic fluorides such as fluorides of sodium, potassium, silver and mercury (I) were described in 1980ties. Though the value of this method of fluorination was discovered by the Belgian chemist Swarts, who used antimony trifluoride as fluorinating agent in 1892. The fluorination by antimony trifluoride became widely spread in the next 60 years.
Until the present time, it was conducted a large quantity reactions of fluorination of C-Cl bond in Hal-C-C systems. The works on fluorination of Hal-C-O systems [1-3] were also carried out. Trichloromethylsulfides with the common formula R-S-CCl2R’ also underwent fluorination by Swarts with antimony trifluoride in the presence of hydrogen fluoride or antimony pentachloride [4,5]. There are a lot of examples in the literature of direct fluorination by Swarts of Cl-P [6-8] and Cl-Si bonds with the formation of tris-, bis- and monofluorination products [9-11]. But there are no examples of direct fluorination of Cl-C-E moiety.
By studying this matter, we discovered a light fluorination of Cl-C-Si system. The value of this achievement is premised on the fact that recently, the number of investigations on the introduction of perfluoroalkyl groups in different functional compounds increased intensively. According to many of them, the one of the most promising source of perfluoroalkyl fragments are perfluoroalkylsilanes. One of the main method of obtainment of silanes is consisted in the usage of perfluoroalkyl bromides as starting compounds. The production of these compounds is prohibited due to their ozone-depleting properties. In this regards, there is an acute need in the sourcing of alternative variants of the preparation of polyfluoroalkylsilanes.
On the bases of the obtained results, we developed the fluorination of (trichloromethyl)trimethylsilane with antimony trifluoride by Swarts. The fluorination is performed in the presence of catalytic quantities of bromine or antimony pentachloride during heating or in inert fluorinated solvent or without solvent (Scheme 1 [12]).
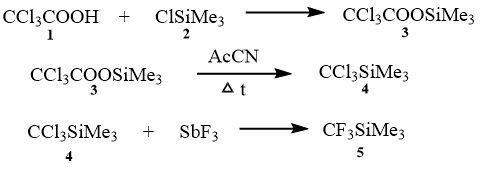
Scheme 1.
Experiment
NMR spectra 1H and 19F were registered in DMSO-d6 and CDCl3 on the spectrometer «Bruker Avance 400» with the working frequency 400,13 MHz and 376,5 MHz accordingly. The chemical shifts 1H are given relatively to TMC (internal standard), 19F relatively to CFCl3- external standard. The constants of the spin-spin interaction are given in MHz.
Trimethyl(trifluoromethyl)silane (5)
Рreparation by fluorination with SbF3+Br2. In a 1L three-neck flask, provided with magnetic stirrer, dropping funnel, thermometer, dephlegmator, connected with Liebig condenser, prolong with Tishchenko flask with concentrated H2SO4 on exit and receiving flask, to 500 ml of p-chlorobenzotrifluoride while stirring, consequently 300 g (1,58 mol) of trimethyl(trichloromethyl)silane and 336 g (1,89 mol) of antimony trifluoride are added. The reaction mixture is heated to the temperature of 100 – 105°C and 28 g (0,176 mol) of bromine are added dropwise, after that the temperature of the still is gradually increased to 138 °C, at the same time the product is collected in the receiving flask, while doing so, the steam temperature shouldn’t increase 65°C. It is obtained 210 g of trimethyl(trifluoromethyl)silane, the purity according to GC and 19F NMR spectrum is 80 %. After rectification, it is obtained 168 g of trimethyl(trifluoromethyl)silane with the purity 99 %. 1H(CDCl3): 0,25 ppm, 19F - 67,5 ppm. The yield is 76 %.
Preparation by fluorination with SbF3+SbCl5. In a 0.5 L three-neck flask, provided with magnetic stirrer, dropping funnel, thermometer, dephlegmator, connected with Liebig condenser, prolong, provided with CO2 condenser with Tishchenko flask with concentrated H2SO4 on exit and receiving flask, 150 g (0,79 mol) of trimethyl(trichloromethyl)silane and 170 g of antimony trifluoride are placed. The reaction mixture is heated to 60 °C and 47 g of antimony pentachloride are added dropwise, after that the temperature of the still is gradually increased to 80- 100 °C, the same time the product is collected in the receiving flask, while doing so, the steam temperature shouldn’t increase 60 °C. The distillate is rectified and 75 g of trimethyl(trifluoromethyl)silane in the form of colorless liquid are obtained. The purity according GC and 19F NMR spectrum is 99 %/ The yield is 68 %.
Conclusion
We described the method of preparation of the synthetically important compound – (trimethyl)trifluoromethylsilane using fluorination by Swarts.
Literature
- Booth H. S., Burchfield P.E., J. Am. Chem. Soc., 1935, 57, 2070;
- Booth H. S., Patent US 2066905, 1937 (Westinghouse Electric and Mfg. Co.) C. A., 1937, 31, 1037;
- Miller C. B., Woolf C., Patent US 2803665, 1957 (Allied Chemical Corp.); C. A. 1958, 52, 2047;
- Yagupol'skij L.M., Marenec M.S., ZhOKh, 1959, 29, 278 (in Russian);
- Truce W.E., Birum G.H., McBee., J. Am. Chem. Soc., 1952, 74,3594;
- Smith W.C., Patent US 2904588, 1958 (E.I. du Pont de Nemours & Co.); C.A., 1960, 54, 2254;
- Booth H.S., Martin D.R., Kendall F.E., J. Am. Chem. Soc., 1948, 70, 2523;
- Schrader G., Bayer O., пат. США 2146356, 1939; С.A., 1939, 33, 3500;
- Booth H.S. Carnell P.H., J. Am. Chem. Soc., 1946. 68. 2650;
- Booth H.S., Spressard D.R., J. Am. Chem. Soc., 1946. 68. 2660;
- Booth H.S., Schwartz A.A., J. Am. Chem. Soc.,1946, 68. 2662;
- S.M. Igumnov, V.E. Boiko, V.L. Don WO2015/142212 A1 2014.
Recommended for publication by V.V. Kornilov
Fluorine Notes, 2018, 120, 7-8
