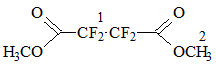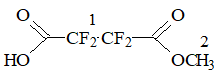Received: November 2018
DOI 10.17677/fn20714807.2018.06.02
Fluorine Notes, 2018, 121, 3-4
ELECTROCHEMICAL FLUORINATION OF MALEIC ANHYDRIDE
N.B. Lesnevskaya, E.V. Litvinenko, A.A. Ludikainen, V.A. Matalin, T.V. Mikhailova
Federal State Unitary Enterprise «Russian Scientific Center «Applied Chemistry», 193232, Russia, St.Petersburg, ul. Krilenko, 26-А
e-mail:matalin.v@yandex.ru, v.matalin@giph.su
Abstract: A novel method is proposed for the manufacture of perfluoropropionyl fluoride and perfluorobutanedioyl difluoride by electrochemical fluorination of maleic acid anhydride. The method offers essential economic and technological advantages over those earlier used in full-scale production of those fluorides.
Keywords: perfluoropropionyl fluoride, perfluorobutanedioyl difluoride, electrochemical fluorination.
Electrochemical fluorination (ECF) is one of important modern-day industrial methods for perfluorinated organic compounds that retains their functionalities unchanged. Efforts to improve the ECF process and to find the most suitable starting materials for the synthesis provide an important practical issue because of unique physico-chemical and performance properties of the resulting fluorinated products. It stimulates researchers to look for new approaches to synthesis and to update the available processes in order to increase the yield of target products and to reduce economic costs.
The objective of this study was to optimize ECF process with respect to the production of alkanoyl fluorides and selection of the best starting materials.
It is well known that perfluoropropionyl fluoride is producible by ECF of propionyl chloride and 2,2,3,3-tetrafluoro-1-propanol, however, the factory cost of the target product is high due both to expensive starting materials and non-optimal process yield. To optimize the process, in this study we investigated the possibilities for the production of some alkanoyl fluorides using maleic anhydride for the starting material. Maleic anhydride is used mainly in the synthesis of polyester resins and some products based on them. Most of those final products are used in construction applications (fiberglass, artificial stone, paintwork) or for raw materials in the production of synthetic rubbers, paints and varnishes, etc. Maleic anhydride is rather cheap, and if used instead of propionyl chloride, e.g., in the synthesis of propionyl fluoride, it provides 6-fold decrease in the cost of raw materials.
The synthesis products have got wide range of applications: perfluoropropionyl fluoride is used as a monomer in the synthesis of composites (ion-exchange membranes, anti-adhesion coatings, optical fibers), or an intermediate in organic synthesis (e.g., in the production of perfluoroethyl isopropyl ketone [1-3]), in the manufacture of emulsifiers, fluorinated surfactants, thermo- and chemically stable liquids, lubricants and polymers [4], while perfluorobutanedioyl difluoride is used in the synthesis of monomers for thermo- and cold-resistant rubbers.
Our study involved the steps as follows:
- Electrochemical fluorination of maleic anhydride, synthesis of perfluoropropionyl fluoride and perfluorobutanedioyl difluoride.
- Separation and isolation of the products by rectification, and identification of its composition and structure by the methods of GLC, and NMR 19F, 1Н spectroscopy.
Experimental
Experimental study involved:
- ECF of maleic anhydride.
- Rectification of crude product, its quality gas-liquid chromatography (GLC) analysis, its identification with NMR 19F-spectroscopy.
ECF of maleic anhydride
The initial concentration of maleic anhydride (MA) was 15% (99g) (mass.). Prior to feeding into an electrolyser MA was dissolved in hydrogen fluoride. The current amperage was 10 A, density 0.02 A/cm2, voltage 5.4-6 V. The electrolyser temperature was 18-26oC, while that of the reflux condenser was minus 36oC.
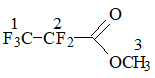
ECF of MA in hydrogen fluoride followed the equation:
Scheme 1
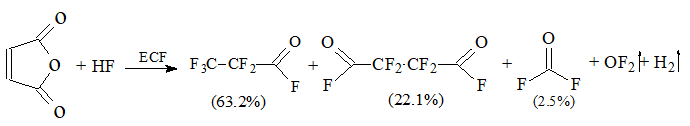
NMR analysis confirmed that ECF of МА resulted mainly in the formation of perfluoropropionyl fluoride (63.2%).
To keep the percentage of fluorinated substance unchanged MA (dissolved in HF) was periodically added into the electrolyser during ECF. Its consumption in ECF process (1) was determined with the help of MA consumption coefficient (C.C.) calculated as per the formula as follows:
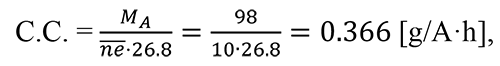
Here: MA is molecular mass of the initial substance;
ne is the number of electrons involved in the reaction;
26.8 is Faraday constant.
The calculated (according to C.C.) amount of MA dissolved in HF was fed into the electrolyser through its metering stopcock so that to fill its sight flow indicator. Then the level of HF was adjusted to the upper edge of the electrodes (control through the upper sight flow indicator). Organic was added in 4-hour intervals, the records were done every half hour.
The electrolyser was charged with 624.6 g of MA, and it was 11 times unloaded during the synthesis; the synthesis time was 206 h; total amount of perfluorinated products (from traps) was 497.6 g. Total electricity consumption was 1871.25 A·h; the related specific electricity consumption was 2835.23 A·h/l.
Current yields were calculated for perfluorobutanedioyl difluoride (DFTSA) и perfluoropropionyl fluoride (PPF).
DFTSA current yield was: η = (422.9∙100%)/(1848.25·0,724)=33.01%
PPF current yield was: η = (481∙100%)/(1848.25·0,516)=50.49%
The related material yields were:
for DFTSA: η = (442.8∙100%)/1236.45=35.81%
for PPF: η= (481∙100%)/1057.99=45.48%
To improve the process performance and yields we conducted similar electrochemical synthesis with addition of triallyl amine. However, the results did not exceed those obtained in MA ECF without additives.
ECF of maleic anhydride. Description of technique
All experiments were conducted in a carbon steel Simons electrolyser (Fig.1) of volume 0.66 l.
Its single-chamber electrolytic cell (1) was equipped with a water-cooled jacket (2). A package of alternating nickel electrodes was attached to the inside of the cell cover (Fig.2). The effective anode area was 502 cm2. The upper cover was equipped with a valve for organic and HF feeding and with a steel reflux condenser (4) cooled to minus 25-32oС with isopropanol, with the help of a circulating thermostat LAUDARP855. The electrolyser bottom had a sight flow indicator with a peep-sight glass (3) and a valve for discharge of crude products.
All gaseous reaction products pass a safety reservoir (6) and two tandem traps (7) charged with methanol and equipped with jackets cooled with isopropanol (to minus 15-20°С) with the help of a circulating thermostat JULABOF33. Fluoroanhydrides and HF are dissolved in methanol while OF2 and H2 are blown off.
The layout of ECF lab-scale plant is shown in Fig.1, the inner design of the electrolyser and traps is shown in Fig.2, and the connection diagram for apparatus and control devices is shown in Fig.3.
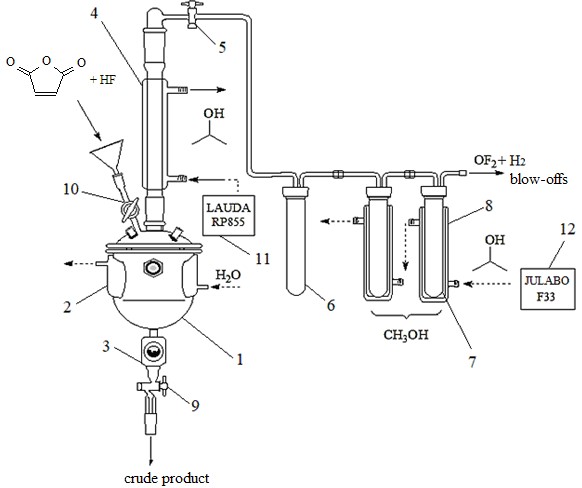
Fig.1 The layout of lab-scale plant intended for ECF of maleic anhydride: 1 – Simons electrolyser; 2 – jacket of the electrolyser; 3 – sight flow indicator; 4 – reflux condenser; 5 – valve for reaction gases feeding into the traps; 6 – safety trap with air; 7 – traps with methanol; 8 – trap cooling jackets; 9 – valve for liquid product discharge (crude product); 10 – valve for reagent feeding; 11,12 – circulating thermostats cooled with isopropanol.
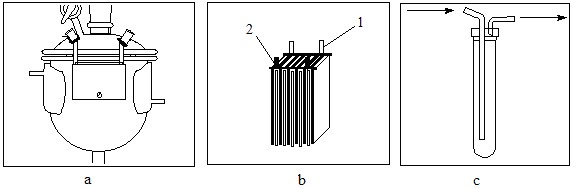
Fig.2 Inner design of the apparatus: a – internal design of the electrolyser; b – package of electrodes; c – internal design of the traps.
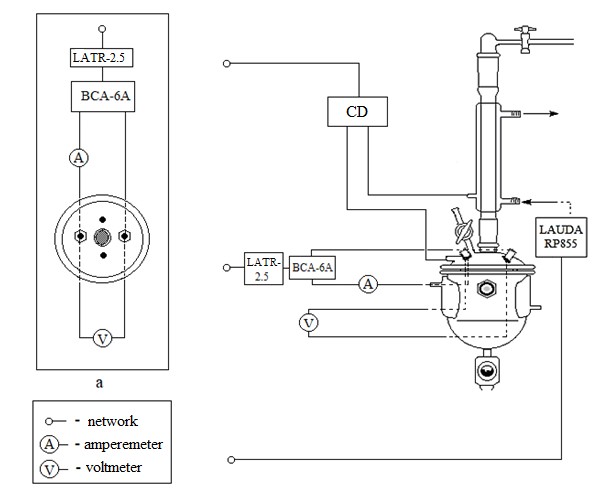
Fig.3 Connection diagram for apparatus and control devices: а – top view; CD – control device; LATR-2,5 – laboratory transformer; ВСА-6А – rectifier; LAUDARP855 – cooling circulating thermostat (thermoregulator).
ECF of maleic anhydride. Product rectification
To retain anhydrides the traps were charged with methanol.
The reaction products underwent esterification with methanol resulting in methyl perfluoropropionate and dimethyl tetrafluorosuccinate:
Scheme 2
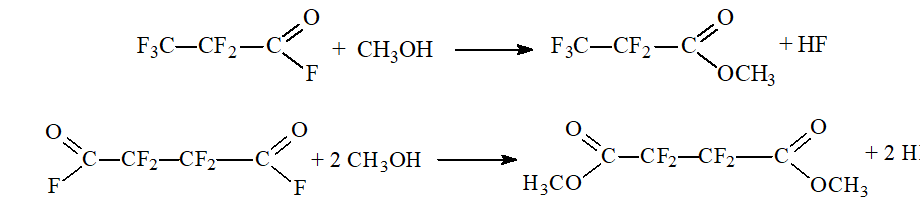
The crude product was discharged and rectified.
The results of rectification are shown in Table 1.
Table 1. ECF of maleic anhydride. Results of the crude product rectification.
|
Fraction # |
BP, °С |
Weight, g |
Product |
|
1 |
47-50 |
6.2 |
Low-boiling impurities |
|
2 |
55-61 |
43.0 |
Methyl perfluoropropionate (С2F5COOCH3). |
|
3 |
71-72 |
2.0 |
Intermediate fraction |
|
4 |
85-90 |
13.8 |
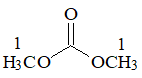
Dimethyl carbonate (H3COC(O)OCH3) |
|
5 |
170-173 |
31.1 |
Dimethyl Tetrafluorosuccinate |
DISCUSSION
We recorded NMR 19F, 1H (LLF543 and LLH543) spectra of ECF crude product (with boiling temperature 170-173°С) dissolved in СDCl3.
The product components were identified by their NMR 19F spectra registered with «Bruker Spectrospin» АМ-500, 470.6 MHz, using deuterochloroform for solvent and hexafluorobenzene for internal standard. The product was GLC-analyzed with Rtx-200 capillary column. The analysis revealed three main products and a number of minor impurities.
Interpretation of NMR spectra and the content of identified substances are shown in Table 2.
Substances I-III were identified, among other methods, by GLC. Identification of IV and V did not contradict NMR results, but required additional confirmation (e.g., by GLC). Non-assigned signals belonged to substances that would be identified precisely under condition of the sample fractionating.
Additional GLC analysis was done with the help of “Kristall-2000M” chromatograph equipped with a thermal conductivity detector, a packed column (2 m), with “silochrome-80”-supported stationary phase (α,α,α-tris(β-cyanethyl)acetophenone, 20% by mass). This additional analysis also confirmed the presence of 3 chief components, their ratio being nearly the same.
Table 2. Interpretation of the identified substances spectra. Content of those substances in the sample resulting from ECF of dimethyl succinate, according to NMR 19F, 1H and GLC.
|
# |
Substance structure |
# at. |
δССl3F, δTMS ppm |
Intensity of lines |
Multiplet |
JF-F*, Hz |
P.S. |
|
|
LLF543 |
LLH543 |
|||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
|
I |
Methyl perfluoropropionate
|
1 |
-83.76 |
43.71 |
s |
|||
|
2 |
-122.22 |
31.47 |
s |
|||||
|
3 |
3.935 |
42.91 |
s |
|||||
|
Intensity of 1 F (1 Н) |
14.6 |
14.3 |
||||||
|
Content, % mol. |
63.2 |
63.3 |
48.7% (GLC) |
|||||
|
II |
Dimethyl tetrafluorosuccinate
|
1 |
-120.61 |
20.27 |
s |
|||
|
2 |
41.74 |
s |
||||||
|
Intensity of 1 F (1 Н) |
5.1 |
7.0 |
Protons are overestimated |
|||||
|
Content, % mol. |
22.1 |
18.3 |
19.6% (GLC) |
|||||
|
III |
Dimethylcarbonate
|
1 |
3.724 |
24.09 |
s |
3.787 ppm data from literature |
||
|
Intensity of 1 Н |
4.0 |
|||||||
|
Content, % mol. |
2.5 |
9.0% (GLC) |
||||||
|
IV |
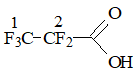
Perfluoropropionic acid
|
1 |
-83.86 |
5.52 |
s |
|||
|
2 |
-122.88 |
3.77 |
s |
|||||
|
Intensity of 1 F |
1.84 |
|||||||
|
Content, % mol. |
8.0 |
|||||||
|
V |
Methyl tetrafluorosuccinate
|
1 |
-120.87 |
1.93 |
t |
J1-2 3.4 |
||
|
2 |
-120.96 |
1.93 |
t |
J2-1 3.4 |
||||
|
3 |
3.901 |
s |
Superimposed with the main signal |
|||||
|
Intensity of 1 F |
0.97 |
|||||||
|
Content, % mol. |
4.2 |
|||||||
|
VI |
Signals of non-identified substances |
1 |
0.77 |
|||||
|
2 |
5.0 |
|||||||
|
3 |
1.23 |
|||||||
|
Intensity of 1 F |
0.6 |
|||||||
|
Content, % mol. |
2.6 |
|||||||
|
Total intensity of 1 F |
23.11 |
|||||||
|
Total, % mol. |
99.9 |
|||||||
CONCLUSIONS
- A new method is proposed for the synthesis of perfluoropropionyl fluoride and perfluorobutanedioyl difluoride by ECF of maleic anhydride. The method excels in economic and technological characteristics those earlier applied in industrial manufacture of those fluoroanhydrides, prepared by ECF of their hydrocarbon analogues, taking into account co-production of the said substances with high yields in the same process.
- The synthesis resulted in production of 498 g of perfluorinated products, the substance yield was 36% and 45% for DFTSA and PPF, correspondingly.
- The products are separated by rectification.
- The mixture composition and the product structure are determined with the help of GLC, and NMR 19F, 1Н spectroscopy.
REFERENCES
- US 4136121 (A). Process for the preparation of fluorine-containing ketones (1979)
- RU 2472767 (C1). Method of producing perfluoroisopropyl ketone (2013)
- RU2494086 (C2) Method of producing perfluoroethylisopropyl ketone (2013)
- Ponomarenko V.A. Fluorine-containing heterochain polymers / V.A. Ponomarenko, S.P. Krukovskiy, A.Yu. Albina. – M.: Nauka. – 1973. – p.118-123
Recommended for publication by Prof. V.G. Barabanov
Fluorine Notes, 2018, 121, 3-4