Received: November 2018
DOI 10.17677/fn20714807.2018.06.04
Fluorine Notes, 2018, 121, 7-8
Co-polymerisation of perfluoro-2-fluorosulfonylethylvinyl ester with other perluoromonomers
E.V. Polunin1*, J.E. Pogodina1, I.A. Prikhno2, A.B. Yaroslavtsev2,3
1 N.D. Zelinsky Institute of Organic Chemistry of Russian Academy of Science, 119991, Leninsky ave., 47, Moscow, Russia
2 Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Science, 119991, Leninsky prosp., 31, Moscow. Russia.
3Institute of Problems of Chemical Physics of the Russian Academy of Science, 142432, Academician Semenov avenue, 1, Moscow region, Russia.
*ep507@mail.ru
Abstract: The new synthesis method of perfluorinated co-polymers on the base of perfluoro-3-oxapent-4-en-1-sulfonylfluoride with perfluorodioxoles, perfluoropropylvinyl ester and perfluoropropene is described. Using hydrolysis on its base there was obtained a material with proton visibility at room temperature and humidity 30% was comparable with such for membrane Nafion.
Keywords: perfluorinated sulfonylfluoride, perfluoromonomers, polymerization under high pressure, thermal initiation, proton-conductive material, proton conductivity.
Proton-conductive membranes are widely applied for various electrochemical technologies [1] and fuel elements are among the most promising directions of its application. Due to the membranes’ severe operating conditions in these devices, chemically stable perfluorinated materials most times are used [2]. The first proton-conductive membranes were obtained in 1966, when the company Du Pont patented a membrane on the base of tetrafluoroethylene and perfluorinated sulfo-containing monomer perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonylfluoride (PSVE, FC-141) [3]. Co-polymer got the commercial name - Nafion. The company Asahi Glass Technology worked out perfluorinated sulfomonomer perfluoro-3-oxahex-5-ene-1-sulfonylfluoride [4] and based on it membranous polymer and Dow Chemical patented sulfo-containing monomer with sulfo-group, hither to double bond, CF2=CFOCF2CF2SO2F (1) [5]. Based on it co-polymer was called Dowlex, but, due to the low reactive capacity of this monomer while co-polymerisation, such membranes failed to be widely used.
According to overage rule, the processes of co-polymerization of perfluorinated sulfo-containing monomers (for example, FC-141) with tetrafluoroethylene are held in fluorocarbon solvent with the usage of different radical initiators (alkylperoxides, perfluorinated alkylperoxides, etc). Although, the big difference in monomers’ reactive capacity, up to two times, is a significant problem when co-polymerization of tetrafluoroethylene with PSVE (FC-141) is held [6,7], and the co-polymerization is been stopped at turning depth ~ 20-25%, after that co-polymer is being washed from unreacted monomer. During this process it is difficult to avoid the losses of expensive sulfo-monomer.
The method of perfluoromonomers polymerization under high pressure with thermal initiation, that allows to react monomers, which were earlier considered to be inert, was developed by Zharov and Guzyaeva [8]. It was shown that under these conditions the difference in monomers’ reactive capacity is smoothed over [9].
According to the stated above, in the purpose of investigation of the possibility of new materials obtainment, for proton-conductive membranes there was held co-polymerization of sulfoether 1 with dioxoles 2 and 3, perfluoropropylvinyl ether 4 and perfluoropropene 5. Proton-conductive material was obtained on the base of tricomponent co-polymer 1+2+4.
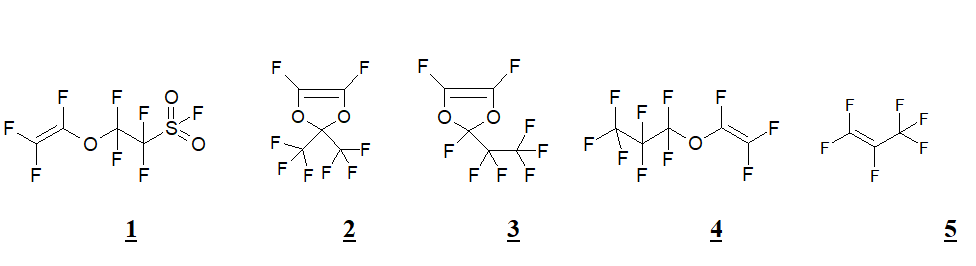
Monomers’ fragments molar ration in co-polymers was found using 19F NMR (300 MHz) based on the ratio of signals integral intensity at +40 ppm (F in sulfonylfluoride) and at ~-79 ppm (trifluoromethyl groups). The sulfomonomer molar ration in binary co-polymers varied from 25 to 45%, in tricomponent co-polymer the ration 1:2:4 was equal to 4:3:3.
Experimental
Monomers - sulfoether 1, dioxoles 2 and 3, and also perfluoropropylvinyl ether 4, previously distilled in an argon atmosphere, in the given molar ratio were placed in cylindrical teflon ampoule with the volume 2 ml and then in «Barostat» [8]. The pressure was graded, 1000 atm during 5 min, raised up to 10000 atm, after that the heating started and the reaction mixture was held during 20 hours at 150 оС. Then the device was cooled down, ampoule was taken out and the product was held under vacuum 2 mbar at 70 оС till the constant mass.
Perfluoropropene 5 was condensated under the argon atmosphere into ampoule with the volume 2 ml (placed in «Barostat» at -70 оС), then there was added 1 in the given molar ratio and the reaction was held at 200 оС as described above.
The co-polymers yield varied from 34 to 60% for binary and 87% for tricomponent co-polymer. Its’ structure and content were proved by 19F NMR.
The hydrolysis of co-polymer sample was held by treating with 0.1М NaOH solution at boiling during two hours with further conversion from natrium form into protonic form while treating with 0.1 М of hydrochloric acid solution. Due to the fact that the films, formed from this polymer were brittle, so the polymer in proton shape was being pressed into a tablet with 6.0 mm diameter under pressure 135 atm during 20 min at room temperature.
The conductivity of the given sample was measured on Elins E-1500 (within the range 1 Hz–1.5 MHz) at the temperature 24°С and relative degree of humidity 30%. The tablet’s resistance value was on the resistance axis and was re-counted into polymer’s conductivity with respect to its geometry, equal 3,8*10-4 Cm/cm. The obtained value was close to Nafion perfluorinated membranes conductivity and to its analogue MF-4CK at the same hymidity[10].
To sum up: in the given article the new synthesis method of perfluorinated co-polymers on the base of sulfoether with dioxoles, perfluoropropylvinyl ether and perfluoropropene was described. Using hydrolysis on its base there was obtained a material with proton visibility at room temperature and humidity 30% equatable to such for Nafion membranes and their analogue MF-4CK.
This work was supported by Russian Science Foundation (grant № 17-79-30054).
References
- Strathmann H., Grabowski A., Eigenberger G. Ind. Eng. Chem. Res. 2013, v.52, p. 10364.
- Stenina I.A., Yaroslavtsev A.B. Pure and Applied Chemistry, 2017, v. 89, p. 1185.
- Pat. 3282875 United States (1966).
- Pat. 5228588 Japan (1977).
- Pat. 4,940,525 United States (1990).
- Odinokov A.S., Bazanova O.S., Sokolov L.F., Barabanov V.G., Timofeev S.V. Russian Journal of Applied Chemistry 2009, v.82, pp. 112-115.
- Ivanchev S.S., Myakin S.V. Russian Chemical Reviews. 2010. v. 79. p. 101-118.
- Zharov A.A., Guzyayeva I.A. Russian Chemical Bulletin, 2010, No. 6, p.1225-1231.
- Sokolov V.I., Boyko V.E., Goryachuk I.O., Igumnov S.M., Molchanova S.I., Pogodina Yu.E. Polunin E.V., Russian Chemical Bulletin. 2017, №7, p. 1284.
- Safronova E.Yu., Stenina I.A., Yaroslavtsev A.B. Russian Journal of Inorganic Chemistry. 2010, v. 55, p. 13-17.
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2018, 121, 7-8
