Received: October 2018
DOI 10.17677/fn20714807.2019.01.02
Fluorine Notes, 2019, 122, 3-4
Electrochemical synthesis of Tb3+ waterless luminescent complex compounds with some fluorine-containing aromatic carboxylic acids
A.I. Oflidi, M.A. Nazarenko, S.L.Kuznetsova
Kuban State University, 350040, Stavropolskaya street, 149, Krasnodar, Russia, e-mail: oflidi@mail.ru
Abstract: Electrochemical synthesis of terbium (III) waterless complex compounds with fluorine-containing aromatic carboxylic acids of TbL3 content was performed. The structure of obtained complex compounds coordination site was studied using the IR-spectroscopy method, and it was found out, that the coordination between used ligands and Tb3+ ions is bidentate. We also studied luminescent properties of the obtained compounds and showed that the terbium (III) with pentafluorobenzoic acid complex compound has the highest luminescent efficiency.
Keywords: electrochemical synthesis, complex compounds, fluorine-containing aromatic carboxylic acids, luminescence.
We have shown earlier [1], that in some cases, d-metals anodic dissolution in various organic compounds solutions is followed by coordination compounds formation. The anodic synthesis is a perspective method, which allows obtaining compounds, sometimes with a different from chemically synthesized structure and properties. The use of electrochemical synthesis provides an opportunity to obtain waterless coordination compounds - what is of great practical interest, for example, when studying coordination compounds of 4f-elements (lanthanides) – perspective luminophores [2]. It is known [2] that the presence of water molecules in lanthanides coordination sphere significantly quenches luminescence and reduces luminophores thermal stability.
Terbium (III) with fluorine-containing aromatic carboxylic acids complex compounds electrochemical synthesis, investigation of its structure and luminescent properties, are the aims of this paper.
Electrochemical synthesis. In order to achieve the synthesis maximum efficiency, it is important to define its optimal conditions and parameters: electrolyte system composition, applied voltage, current rate, current density, temperature, current yield. Considering that in the papers [3,4] it was shown that the neglecting by detailed electrochemistry in waterless solvents does not prevent from individual complexes synthesis, so we did not study volt-ampere characteristics and processes.
During the synthesis of terbium(III) complex compounds with the used ligands, the optimal current density was equal to 0,007-0,013 A/cm2. In order to achieve the optimal current density, the electrochemical cell was imposed by voltage 5 – 9 Volt. Decrease of the current density leads to the process deceleration. If the current density is higher, then the solution heating is possible, what can lead to side reactions during which the target product either is destructed or substantively polluted (for example: anode severe erosion is possible). Due to this reason, the synthesis process was held at the room temperature. In order to achieve high solution conductivity, a background electrolyte, in the ratio 1/10 from quantity of the used ligand, was added in case of need. Lithium perchlorate (LiClO4), which is inert, was used as background electrolyte.
During the complexes synthesis, the formed slightly soluble complex compound adhesion on anode took place and this led to the anode passivation. There was an increase in system electric resistance that led to decrease in the general conductivity. The synthesis process significantly slowed down due to the decrease in current density almost to zero. In order to solve the arose problems, during the synthesis, electrochemical cell was exposed by ultrasonic treatment and, thanks to that, there was a considerable decrease in anode passivation and stabilization of the synthesis processes.
IR-spectroscopy. The comparison of IR-spectrum of obtained coordination compounds with IR-spectrum of initials of aromatic carboxylic acids shown that the last in complexes are in ionized form, because there appears absorption bands of asymmetrical and symmetrical oscillations of deprotonated carboxylic group in the field 1650-1510 cm-1 and 1440-1370 cm-1 correspondingly, and there disappears absorption bands in the field 1665-1700 cm-1, which refers to valence vibrations of C=O bond of nonionized carboxyl group.
The comparative results of Δν(COO-) for Tb3+ complex compounds have shown that the difference between ionized carboxyl group asymmetrical and symmetrical oscillations Δν(COO-) is less than 220 cm-1, what allows to assume its bidentate coordination in a majority of obtained complex compounds.
Coordination compounds luminescence. The obtained terbium (III) complex
compounds have luminescent properties (picture 1), that are typical for Tb3+ ion (green luminescence). At the same time, the fact, that there is no organic ligand phosphorescence,
may prove good energy redistribution from organic ligand to Tb3+ ion. In photoluminescence
spectra we can observe typical for Tb3+ ion emission bands: 5D4→
7F6 (490 nm), 5D4→ 7F5 (545 nm),
5D4→ 7F4 (585 nm), 5D4→ 7F3 (620 nm), 5D4→ 7F2 (650 nm).
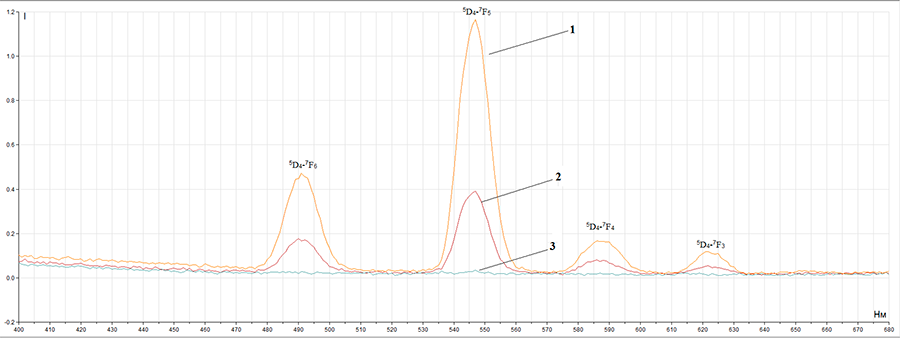
Picture 1 – Complex compounds luminescence spectrum (1 –Tb3+ complex with pentafluorobenzoic acid, 2 – Tb3+ complex with 2,3,4,5-tetrafluorobenzoic acid, 3 – Tb3+ complex with 4-methyltetrafluorobenzoic acid).
It was found out, that terbium (III) with pentafluorobenzoic acid complex compound has the highest luminescence what makes it a perspective luminescent material. At the same time, there is an energy transfer from the initiated ligand triplet level to the terbium (III) ion resonance level without phosphorescence of a complex compound organic part.
Experimental
In the process were used – metal terbium in plates (purity – 99,9%), aromatic benzoic acids: pentafluorobenzoic acid chemically pure, 2,3,4,5-tetrafluorobenzoic acid chemically pure, 4-methyltetrafluorobenzoic acid chemically pure.
Direct electrochemical synthesis of complex compounds was held using the method of the soluble anode with the usage of a direct current source in a two-electrode cell.
The cell (picture 2) consists of a glass reactor with densely grinded top in which electrodes – the terbium anode and the platinum cathode are located; on the bottom of the cell magnetic mixer anchor, for constant solution stirring, is placed.
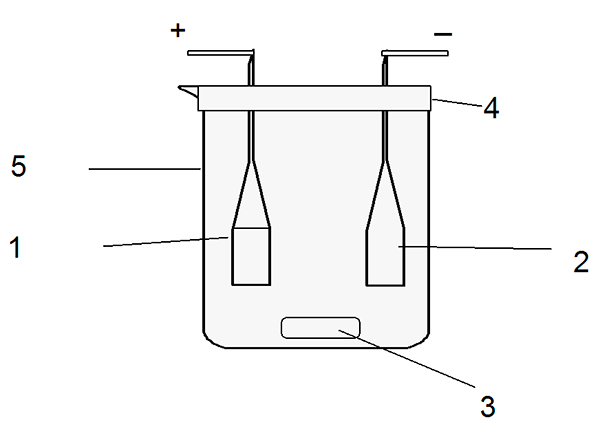
Picture 2 - Electrolytic cell scheme: 1. Anode – working electrode; 2. Cathode – auxiliary electrode; 3. Magnetic mixer anchor; 4. Hermetic cover; 5. Glass reactor.
The dehydrated acetonitrile was used as nonaqueous solvent at electrochemical synthesis. Time of synthesis was determined, proceeding from initial concentration of ligands by Faraday's law and varied from 2 to 3 hours. Processes were held in the inert atmosphere in tight system.
After the electrochemical synthesis was finished, the white color slightly soluble complexes which precipitated out, were filtered on the Shota filter, were washed with acetonitrile and dried in the vacuum furnace at temperature 30-50◦С.
The terbium (III) content in the obtained complex compounds was calculated by the complexometric titration method.
The carbon and hydrogen content was determined by method of the element microanalysis on C,H,N,S-analyzer VARIO MICRO CUBE in oxygen current at a temperature of furnace - 1200 °C.
The complex compounds analysis data show that the complexes content corresponds to the TbL3 general formula.
Complexes and ligands IR-spectra were recorded on IR-Fourier spectrometer VERTEX 70 (Bruker) in 4000-400 cm-1 field. Solid, using attenuated total inside reflection adaptor with diamond crystal.
Ranges of luminescence initiation and registration were recorded at room temperature on a spectrofluorimeter the Flyuorat-02-Panorama (Lumex). Solid samples of obtained complex compounds were used for recording.
Conclusions
1. Using the electrochemical synthesis methods, we have shown the possibility in principle for obtainment of terbium (III) with fluorine-containing aromatic carboxylic acids waterless complex compounds.
2. Using the IR-spectroscopy analysis method, the bidentate way for coordination of fluorine-containing carboxylic acids with Tb3+ ions was established.
3. It was found out that terbium (III) with pentafluorobenzoic acid complex compound has the highest luminescence.
Literature
- Frolov V.Yu., Oflidi A.I., Bolotin S.N., Shestavin A.I., Panyushkin V.T. // Russ. J. Appl. Chem., 2008, Vol. 81, No 4, P. 639, DOI: 10.1134/S1070427208040137.
- Katkova M.A., Vitukhnovsky A. G., Bochkarev M. N. // Russ. Chem. Rev., 2005, Vol. 74, No 12, P. 1089, DOI: 10.1070/RC2005v074n12ABEH002481.
- Konev, V.A., Kukushkin V.Yu., Kukushkin Yu.N. // Rus. J. Inorg. Chem. 1996, V. 41, No 9, P. 1466.
- Said F.F., Tuck D.G. // Canad. J. Chem., 1981, Vol. 59, P. 62.
Recommended for publication by Prof. S. M. Igumnov
Fluorine Notes, 2019, 122, 3-4
