Received: December 2018
DOI 10.17677/fn20714807.2019.01.04
Fluorine Notes, 2019, 122, 7-8
Chlorin Type Photosensitizers Soluble in Perfluorocarbons: Synthesis and Properties
aBelyaeva E. V.*, aMarkova A. A., aZakharko M. A., bRadchenko A. S., aSigan A. L., aIkonnikov N. S., сShtil A. A., aChkanikov N. D.
a A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 Vavilov Street, GSP-1, Moscow, Russian Federation
E-mail: faftor.belyaeva@mail.ru
b N. M. Emanuel Institute of Biochemical Physics Russian Academy of Sciences, 4 Kosygin Street, Moscow, Russian Federation
c N. N. Blokhin National Medical Research Center of Oncology, 24 Kashirskoe shosse, Moscow, Russian Federation
Abstract: The approach for synthesis of previously unknown polyfluorinated chlorins has been developed. Optical characteristics, solubility in perfluorodecalin and generation of reactive oxygen species by these compounds were investigated. Obtained compounds are considered for potential application as photosensitizers within perfluorocarbon emulsions in photodynamic therapy of cancer.
Keywords: porphyrins, chlorins, photosensitizers, azomethine-ylides cycloaddition, fluorocarbons, perfluorodecalin, solubility in fluorocarbons, reactive oxygen species.
Introduction
Photosensitizers (PS) are used in photodynamic therapy (PDT) of cancer to generate reactive oxygen species (ROS). One perspective direction is the application of PS in combination with fluorocarbon emulsions [1]. Previously we have reported the Perftoran based fluorocarbon emulsions containing polyfluorinated porphyrins as potent PS. These emulsions demonstrated the ability to kill cultured tumor cells in hypoxia (0.5% O2) upon light activation whereas the free form of PS (no emulsion) was inactive [2]. This efficacy has been attributed to the ability of fluorinated liquids to carry oxygen.
Chlorins, the hydrogenated derivatives of porphyrins, are preferable scaffolds for the design of PS. Unlike porphyrins, chlorins absorb light in the longer wavelength region (630–800 nm) that allows for deeper tissue damage. We sought to take advantage of the chlorin macrocycle as a scaffold for fluorination in an attempt to make a series of red shifted PS capable of being efficacious in the conditions of limited oxygen consumption. The reported examples of synthesis of highly fluorinated chlorins soluble in fluorocarbons are reduced to the modification of natural chlorins [3,4]; the number of obtained compounds remains limited. In this study we developed a convenient synthetic method for polyfluorinated chlorins and determined their characteristics relevant for PS such as optical properties, solubility in perfluorodecalin (PFD, a major fluorocarbon component of Perftoran) and the efficacy of light induced ROS generation.
Results and discussion
Synthesis: The first example of chlorin synthesis by [3+2]-cycloaddition of azomethine-ylide to the 'quasi-isolated' double bond of the meso-tetrakis(pentafluorophenyl)porphyrin was demonstrated by Silva et al. [5]. The reaction mechanism involved stepwise formation of azomethine-ylide from the aldehyde and α-amino acid, and the addition of this highly reactive intermediate to the pyrrole fragment of the porphyrin ring. Among the reaction products the starting porphyrin, mono- (chlorin), bis- (isobacterio- and bacteriochlorin) and tris-adducts were detectable.
In present work we used the previously obtained meso-aryl- (1a-c and 1e) and meso-alkyl- (1d) porphyrins as starting materials [2, 6]. Azomethine-ylide was generated from paraformaldehyde and N-methylglycine. Reaction conditions and main products are shown in Scheme 1.
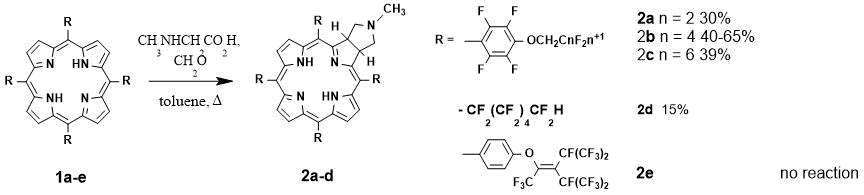
Scheme 1. The 1,3-dipolar cycloaddition of azomethine-ylide to porphyrins 1a-e.
1,3-Cycloaddition of azomethine-ylide to meso-aryl-substituted porphyrins 1a-c with linear perfluoroaliphatic chains yielded the corresponding chlorins 2a-c in 30-65% yields. Regardless of the reaction duration and the ratio of reagents the starting porphyrin always remained in the reaction mixture, while the elongation of reaction time led to an increase of only bis- or tris-adduct fraction. Contrary to expectations, the meso-aryl-substituted porphyrin 1e with a bulk perfluoro substituent did not react with azomethine-ylide. On the UV-Vis spectra of the reaction mixture only the signals of the starting porphyrin were observed. 1,3-Cycloaddition of azomethine-ylide to meso-alkyl-substituted porphyrin 1d gave the corresponding chlorin 2d in a small amount (< 15%). Of note, only a few non-fluorinated analogs of meso-alkyl-substituted chlorins have been obtained. Among the synthetic methods the lithiation with alkyl lithium (this reaction proceeds non-selectively via β- and meso-positions with violation of aromaticity) [7], reduction with p-tosylhydrazine [8] and oxidation with osmium tetroxide [9] have been used.
Comparison of reactivity of 1a-e shows that the presence of perfluoroaliphatic groups (bulky or located close to the macrocycle) blocked the porphyrin reaction center ('quasi-isolated' double bond). Azomethine-ylide as a charged dipole is hardly involved into [3+2]-cycloaddition.
Optical parameters: The position of the absorption maximum and the molar extinction
coefficient value in the region 630–800 nm is a key property of PS. Absorption spectra for porphyrin
1b and the corresponding chlorin 2b clearly demonstrate the advantages
of chlorins - chlorin 2b absorbs light in the longer wavelength region significantly
better than porphyrin 1b (Figure 1).
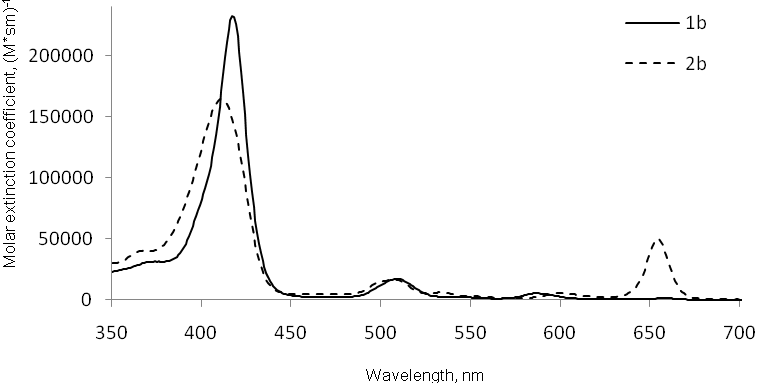
Figure 1. Absorption spectra of porphyrin 1b (solid line) and chlorin 2b (dashed line) in benzene.
Optical characteristics of 1a–c and 2a–c were also studied in PFD solutions. We showed that the perfluoroaliphatic chain length has no significant effect on the position of absorption maxima in both series of chlorins and porphyrins. For chlorins the batochromic shift of Q4 band relative to porphyrins was 4-5 nm, and the molar extinction coefficient of Q4 band was one order of magnitude higher than that of the corresponding porphyrins (see Table 1).
Table 1. Optical parameters, ROS generation efficacy and solubility in PFD of starting porphyrins 1a-d and the corresponding chlorins 2a-c.
|
No |
Absorption maxima λ, nm (ε x 10-3, (M x cm)-1) |
ROS generation efficiency |
Solubility in PFD cmax, mM |
||||
|
Соре |
Q1 |
Q2 |
Q3 |
Q4 |
|||
|
1a |
407 |
503 (22.5) |
533 (2.2) |
582 (6.4) |
653 (1.0) |
- |
1 |
|
1b |
407 |
502 (17.9) |
532 (1.9) |
582 (5.0) |
653 (1.7) |
- |
1 |
|
1c |
406 (137.2) |
501 (13.9) |
* |
583 (4.7) |
654 (2.3) |
0.83** |
1 |
|
1d |
398 (89.4) |
505 (9.1) |
537 (6.5) |
595 (3.7) |
647 (9.3) |
0.72** |
1 |
|
1e |
410 |
507 |
539 |
587 |
643 |
0.91** |
1 |
|
2a |
400 (173.9) |
494 (14.6) |
502 (17.0) |
598 (4.9) |
650 (61.9) |
0.54 |
2 |
|
2b |
399 (147.6) |
494 (12.6) |
502 (14.6) |
596 (4.6) |
650 (50.4) |
0.50 |
114 |
|
2c |
400 (189.9) |
493 (17.2) |
502 (19.3) |
* |
650 (65.3) |
0.48 |
>100 |
*impossible to determine the position of the maximum absorption due to the overlap of bands; **singlet oxygen phosphorescence measured in benzene.
Solubility: To study the solubility of fluorinated porphyrins and the corresponding chlorins in PFD we used spectrophotometry. Table 1 shows that, in contrast to almost insoluble in PFD meso-tetrakis(pentafluorophenyl)porphyrin (<1 μM), the solubility of porphyrins 1a-e was ~ 1 mM; this parameter did not depend on the structure of perfluoroaliphatic substituents. Significant variations were observed for chlorin derivatives: differential solubility of 2a-c varied within 2 orders of magnitude and depended on the perfluorocarbon chain length (Table 1). This trend may be associated with the removal of N-methylpyrrolidine fragment of chlorin from the macrocycle plane, thereby influencing the packing density and the binding energy of molecules in the crystal structure.
ROS generation: ROS are a major factor of cell and tissue photodamage in PDT. The chemical trap method allows for ROS measurement by using the compounds that react completely and irreversibly with ROS. For measurements in non-polar solvents the most common trap is 1,3-diphenylisobenzofuran (DPIBF), whose concentration can be calculated from the absorption spectra. As a standard in calculating ROS we used the closest structural analog of the studied PS, meso-tetrakis(pentafluorophenyl)porphyrin. Its ROS generation efficacy in benzene is 0.7 according to the literature data [10]. Table 1 shows the values of relative ROS generation efficacy in benzene for chlorins 2a–c (Table 1; see also the values of singlet oxygen phosphorescence for starting porphyrins 1c-e relative to the same standard [2]).
Conclusion
As a result an approach for obtaining polyfluorinated chlorins by [3+2]-cycloaddition of azomethine-ylide to the 'quasi-isolated' double bond of the porphyrin ring is developed. In comparison with the corresponding porphyrins, the obtained chlorins are well soluble in fluorocarbons. And due to their optical characteristics and ability to generate ROS, this compounds can be considered perspective for application as a PSs within fluorocarbon emulsion systems for PDT.
Experimental section
Equipment: Spectra NMR 1H and 19F were recorded on a Bruker AMX-400 and AMX-300 spectrometer with frequency 400.13 and 376.50 MHz at 20 °С. Chemical shifts of 1H and 19F were determined relatively to proton signal of solvents (CDCl3, dmso-d6) and trifluoroacetic acid (TFA) as external standard. The ESI (1) and APCI (2) mass-spectra were registered on the Finnigan LCQ Advantage tandem dynamic mass-spectrometer. Nitrogen 10/0 (1) or 70/10 (2) served as a sheath and auxiliary gas. Flow rate of acetonitrile 50 µl/min (1) or 350 µl/min (2).The temperature of the heated capillary was 150 °С, the electric potential between the needle and the counter electrode was 4.5 kV (1) or 6.0 kV (2). The samples with the concentration of 10-4 mol/l in acetonitrile solution were introduced into the ion source through the Reodyne injector with the 5 µl loop. Monitoring of the reaction course and purity of products were controlled by Merck Kieselgel 60 F254 plates. For column chromatography silica gel (MN Kieselgel 60) was used. Absorption spectra were registered on double-beam spectrophotometer Cary-300 UV-Vis (Agilent, USA) and single-beam spectrophotometer Beckman DU 68 (Beckman Instruments, Japan) at room temperature in perfluorodecalin and benzene (scan window – 350-850 nm, in quartz cuvette 1×1 cm).
Reagents and solvents: Toluene, benzene, chloroform, ethyl acetate were distilled before using. Perfluorodecalin (P&M Invest), 1,3-diphenyl-isobenzofuran (Sigma-Aldrich) and other reagents (Acros Organics) were used without additional purification.
General procedure for synthesis of chlorins: To a solution of porphyrin 1a-e (0.10 g, 1 eq.) in toluene (25 ml) N-methylglycine (2 eq.) and paraformaldehyde (4.7 eq.) were added. Resulting mixture was refluxed for 5 hours under argon atmosphere. Depending on the reactivity of substrates, additional portions of N-methylglycine (2 eq.) and paraformaldehyde (4.7 eq.) were added and the reaction mixture was refluxed for another 5 h period. Then reaction mixture was evaporated under reduced pressure and residue was purified by column chromatography on silica gel with gradient elution (chloroform - ethyl acetate).
2a (2,3,3а,21а-tetrahydro-2-methyl-5,10,15,20-tetrakis[2,3,5,6-tetrafluoro-4-(1H,1H-perfluoropropyl-1-oxy)phenyl]-1Н,23Н,25Н-pyrrolo[3,4-β]porphin). Dark purple powder with green shimmer, yield 30%. NMR 1H (CDCl3 (7.28), δ, ppm): -1.86 (s, 2H, NH), 2.74 (s, 3H, CH3N), 3.06 (s, 2H, CH2N), 4.19 (s, 2H, CH2N), 4.95-5.09 (m, 8H, CH2CF2), 5.78 (s, 2H, CH), 8.38 (m, 2H, Pyr-H), 8.55 (s, 2H, Pyr-H), 8.78 (m, 2H, Pyr-H). NMR 19F (CDCl3, from TFA, δ, ppm): -80.45 - -80.18 (m, 4F), -79.06 (m, 2F), -77.30 (m, 2F), -62.05 (m, 2F), -61.70 (m, 4F), -60.99 (m, 2F), -48.55 (m, 8F), -7.53 (m, 12F). UV-Vis λmax, nm (ε x 103, (cm x M)-1) in benzene: 411 (176.1), 507, 534, 600, 654 (53.8); in PFD: 400 (173.9), 494 (14.6), 502 (17.0), 598 (4.9), 650 (61.9). ESI-MS, m/z: 1551.9 [M+H]+.
2b (2,3,3а,21а-tetrahydro-2-methyl-5,10,15,20-tetrakis[2,3,5,6-tetrafluoro-4-(1H,1H-perfluoropentyl-1-oxy)phenyl]-1Н,23Н,25Н-pyrrolo[3,4-β]porphin): Dark purple powder with green shimmer, yield 65%. NMR 1H (CDCl3 (7.28), δ, ppm): -1.93 (s, 2H, NH), 2.73 (s, 3H, CH3N), 3.08 (s, 2H, CH2N), 4.19 (s, 2H, CH2N), 5.00-5.13 (m, 8H, CH2CF2), 5.78 (s, 2H, CH), 8.38 (m, 2H, Pyr-H), 8.55 (s, 2H, Pyr-H), 8.79 (m, 2H, Pyr-H). NMR 19F (CDCl3, from TFA, δ, ppm): -80.45, -80.17, -79.06, -77.28, -62.13, -61.74, -60.98, -50.49, -48.25, -45.00, -5.08. UV-Vis λmax, nm (ε x 103, (cm x M)-1) in benzene: 411 (162.5), 507 (17.2), 534 (6.6), 600 (6.0), 654 (49.6); in PFD: 399 (157.8), 494 (12.6), 502 (14.6), 596 (4.6), 650 (50.4). APCI-MS, m/z: 1952.4 [M+H]+.
2c (2,3,3а,21а-tetrahydro-2-methyl-5,10,15,20-tetrakis[2,3,5,6-tetrafluoro-4-(1H,1H-perfluoro-heptyl-1-oxy)phenyl]-1Н,23Н,25Н-pyrrolo[3,4-β]porphin): Dark purple powder with green shimmer, yield 39%. NMR 1H (CDCl3 (7.28), δ, ppm): -1.93 (s, 2H, NH), 2.70 (s, 3H, CH3N), 3.05 (s, 2H, CH2N), 4.18 (m, 2H, CH2N), 4.98-5.12 (m, 8H, CH2CF2), 5.75 (s, 2H, CH), 8.37 (m, 2H, Pyr-H), 8.55 (s, 2H, Pyr-H), 8.78 (m, 2H, Pyr-H). NMR 19F (CDCl3, from TFA, δ, ppm): -80.36, -79.10, -77.35, -62.11, -61.77, -61.07, -50.41, -47.26, -47.05, -46.34, -44.76, -42.72, -5.03. UV-Vis λmax, nm (ε x 103, (cm x M)-1) in benzene: 411 (215.3), 507 (21.9), 534 (8.1), 600 (7.4), 654 (61.8); in PFD: 400 (189.9), 493 (17.2), 502 (19.3), 596, 650 (65.3).
2d (2,3,3а,21а-tetrahydro-2-methyl-5,10,15,20-tetrakis[6-H-perfluorohexyl-1]-1Н,23Н,25Н- pyrrolo[3,4-β]porphin): Dark purple powder, yield 15%. NMR 1H (dmso-d6 (2.51) + CDCl3 (7.28), δ, ppm): 2.05 (s, 3H, CH3N), 3.09 (s, 2H, CH2N), 3.46 (s, 2H, CH2N), 5.57 (s, 2H, CH), 6.19-6.61 (m, 4H, CF2H), 8.31-8.94 (m, 6H, Pyr-H). NMR 19F (dmso-d6 + CDCl3, from TFA, δ, ppm): -136.94, -129.35, -123.22, -121.37, -115.34, -86.96. UV-Vis λmax, nm in benzene: 407, 506, 687. APCI-MS, m/z: 1568.8 [M+H]+.
Solubility in PFD: To determine the solubility, saturated at room temperature solutions of porphyrins 1a-e and chlorins 2a-c in PFD were prepared. To speed up the dissolution, the process was carried out with heating, then the solutions were kept at room temperature for several days to achieve equilibrium. Saturated solutions were diluted for accurate measurement of optical density (using a range from 0.1 to 1.0 units). Absorption spectra were recorded on a Beckman DU 68 spectrophotometer. The concentration of porphyrins 1a-e and chlorins 2a-c in solutions was calculated with using of known extinction coefficients in accordance with the Bouguer-Lambert-Beer law.
Reactive oxygen species (ROS) generation: In the experiments freshly prepared solutions of standard PS (meso-tetrakis(pentafluorophenyl)porphyrin), trap (1,3-diphenyl-isobenzofuran - DPIBF) and chlorins 2a-c in benzene were used. Concentrations of all PSs were about 10-4 M, DPIBF - 10-3 M. Irradiation of the solutions was performed on a Fluorolog-3 spectrofluorometer (HORIBA Scientific, Japan) with a Xenon lamp 450 W as light source. The excitation wavelength for all samples was 503 nm, the analytical wavelength for monitoring the concentration of DPIBF was 440 nm. Absorption spectra were recorded on a Cary-300 spectrophotometer.
Procedure: 2.5 ml of benzene, 100 μl of the PS solution and 50 μl of the DPIBF solution were placed into a quartz cuvette (1x1 cm) equipped with a stirring device. Resulting solution was irradiated (λ = 503 nm) for 15 s 3-4 times. After every irradiation the absorption spectrum was recorded. Then the plot of the absorbance of the solutions (at λ = 440 nm) versus the irradiation time was built, the boundaries of the linearity region were determined, and the slope of the linear part (α) was calculated. Efficacy of ROS generation was calculated by Formula 1.
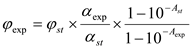 (1),
(1),
where ψ&exp and ψst - ROS generation effectiveness for studied PS and for standard PS (according for literature data), αexp и αst - the slope of the linear part of the graph A-t at λ = 440 nm, Aexp and Ast - optical density of studied PS solution at excitation wavelength (503 nm).
Acknowledgments
The contribution of Center for molecule composition studies of INEOS RAS is gratefully acknowledged.
This work was supported by the Russian Foundation for Basic Research (No 18-315-00432)
References
- Scheer A., Kirsch M., Ferenz K.B. Perfluorocarbons in photodynamic and photothermal therapy // J. Nanosci Nanomed. 2017. Vol. 1. No. 1. P. 21–27.
- Belyaeva E. V. et al. Novel fluorinated porphyrins sensitize tumor cells to photodamage in normoxia and hypoxia: synthesis and biocompatible formulations //Anticancer Agents Med. Chem. 2018. Vol. 18. No. 4. P. 617–627.
- Shibata R. et al. Self-aggregation of synthetic zinc chlorophyll derivatives possessing multi-perfluoroalkyl chains in perfluorinated solvents. // Photochem. Photobiol. Sci. 2007. Vol. 6. No. 7. P. 749–757.
- Tamiaki H., Nishiyama T., Shibata R. Self-aggregation of zinc chlorophylls possessing perfluoroalkyl chains in fluorous solvents: Selective extraction of the self-aggregates with fluorous phase and accelerated formation of the ordered supramolecules in this phase. // Bioorg. Med. Chem. Lett. 2007. Vol. 17. No. 7. P. 1920–1923.
- Silva A.M.G. et al. meso-Tetraarylporphyrins as dipolarophiles in 1,3-dipolar cycloaddition reactions // Chem. Commun. 1999. No. 17. P. 1767–1768.
- Belyaeva E. V. et al. A method of introducing fluorinated substituents in porphyrin structure by nucleophilic substitution of fluorine in meso-tetrakis(pentafluorophenyl)porphyrin and pentafluorobenzaldehyde with polyfluoroaliphatic alcohols // Fluorine notes: internet journal 2015, N 5(102), URL: http://notes.fluorine1.ru/public/2015/5_2015/letters/rusletter2.html
- Sergeeva N.N. et al. Synthesis of hydroporphyrins based on comparative studies of palladium-catalyzed and non-catalyzed approaches. // Tetrahedron. 2007. Vol. 63. No. 50. P. 12454–12464.
- Ikezaki A. et al. Electronic structure of low-spin six-coordinate iron(III) meso -tetrapropylchlorin complexes // J. Porphyr. Phthalocyanines. 2014. Vol. 18. No. 08-09. P. 778–791.
- Aicher D. Preparation of β-functionalized dihydroxy-chlorins for photodynamic therapy. / D. Aicher, A. Wiehe, C. B. W. Stark, V. Albrecht, S. Grafe // PCT Int. Appl. –2012. –No WO2012012809A2 –53pp.с.
- Grancho J.C.P. et al. Synthesis, Spectra and Photophysics of some Free Base Tetrafluoroalkyl and Tetrafluoroaryl Porphyrins with Potential Applications in Imaging. // Photochem. Photobiol. 2002. Vol. 75. No. 3. P. 249.
Recommended for publication by Prof. S. M. Igoumnov
Fluorine Notes, 2019, 122, 7-8
