Received: February 2019
DOI 10.17677/fn20714807.2019.02.02
Fluorine Notes, 2019, 123, 3-4
Obtainment and thermal characteristics of the composition of perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid triethoxysilylpropylamide (Fluorosam-39) and fluororubber SKF-32
Sakharov А.М., Popovich M. J., Krukovsky S.P.
N.D. Zelinsky Institute of Organic Chemistry Russian Academy of Science, 117991, Moscow, Leninsky prospect, 47, tel.: (499)135 63 79,
E-mail: *yar@ioc.ac.ru, as@zelinsky.ru
Abstract: A new methodology for obtainment of compositions based on Fluorosam-39 monomer (triethoxysilylpropylamide perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid) with fluororubber SKF-32 (vinylidene fluoride with trifluorochloroethylene co-polymer) in the presence of AMEO (γ- aminopropyltriethoxysilane) as a catalyst and co-reactant was developed. Content of fluororubber in compositions varied from 10 to 55% (mass.). Thermal properties of the composition, obtained from Fluorosam-39:SKF-32:AMEO at the ratio 45,5: 48,5: 6 (mass.%) were measured using thermomechanical and thermogravimetric analysis methods.
Keywords: Fluorosam-39 monomer, Fluorosam-39 polymer, fluororubber SKF-32, AMEO, TMA, TGA.
Due to the complex of valuable properties, polysiloxanes find more and more broad application in various technical and technology fields. The variation of structure and properties of the macromolecules of the given organosilicone polymers can contribute to a substantial expansion of boundaries of their application in a composition of new hybrid materials of different designation [ 1, 2 ].
Products, based on polysiloxanes, provide high thermal stability, exterior resistance, oxygen and ozone resistance at elevated temperatures, radiation resistance, non-toxicity and biological inertness, high insulation properties. It is considered that high thermal stability of organosilicone polymers is first of all caused by high Si-O binding energy [3, 4].
Fluororubbers are of utmost interest among high heat resistant polymers. The outstanding features of fluororubbers are the high thermal resistance, exceeding the heat resistance of all known rubbers (except siloxanes), chemical inertness, stability, durability, noncombustibility. Fluororubbers contain from 53 to 70% fluorine atoms, resulting in their resistance to aging at high temperatures, to influence of different oils, petrol and other aggressive reagents and are therefore widely used in various technical fields [5].
The possibility of combining of perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid triethoxysilylpropylamide (Fluorosam-39) with fluororubber SKF-32 is examined in this work.
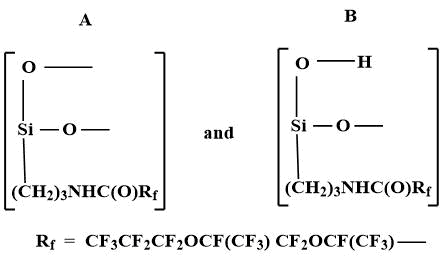
As a result of the reaction of Fluorosam-39 with atmospheric moisture (20-100 °C) colorless, transparent, amorphous, brittle polymers with Tg = 200-230 °C and Tf =250-270°C are formed. Polymer F-39 contains 54.1% of fluorine atoms and 4.7% of silicon atoms. It is a polysiloxane of branched structure, included units А and B [6].
In order to obtain different products, based on Fluorosam-39, it should be modified for reduction of glass transition point and for alleviation of further treatment. Fluororubber SKF-32, that contains 54-55% of fluorine atoms, was used as a modificator, AMEO (γ- aminopropyltriethoxysilane) was used as a catalyst and co-reactant. The composition and structure of the obtained polymer depend on the ratio of starting compounds. Fluororubber content in compositions varied from 10 to 55% (mass. %). The samples were obtained as films with different thickness.
According to TMA data (pic.1) the SKF-32 glass transition temperature is -17 °C, the flow temperature is 300 °C. At about 350 °C we can observe low-grade transition, which is probably due to the partial decomposition of the rubber. At 370-380 °C 100% sample deformation happens. TMA data for compositions of F-39:SKF-32:AMEO=45.5: 48.5: 6 (mass.%) are shown in the pic. 2. The composition’s glass-transition temperature (-20°C) and glass-transition temperature of SKF-32 (-17°C) are close and are lower than glass-transition temperature of Fluorosam-39 (200-230 °C). The flow temperatures of SKF-32 and the composition are 300 °C and 288 °C correspondingly and are nearly similar to Tf of the F-39 polymer (250-270 °C). Both in the rubber SKF-32 and in all compositions where it is used, we can observe low-grade transition at 30-42 °C, which intensity is much lower than Tg and Tf.
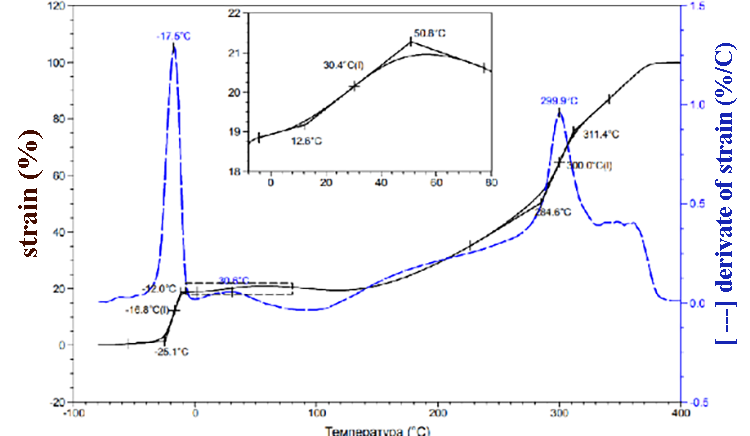
Pic 1. SKF-32 TMA data.
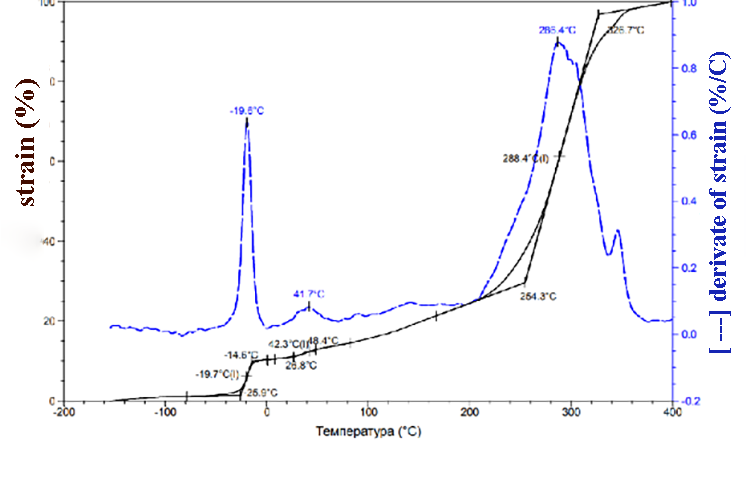
Pic. 2. TMA data of the composition F-39: SKF-32: AMEO=45.5: 48.5: 6 (mass.%).
The combining of polymer F -39 with SCF-32 was carried out by the reaction of monomer F -39 with rubber in the presence of AMEO. In fact, grafting of the monomer f -39 to the rubber was carried out by the following likely scheme [7].
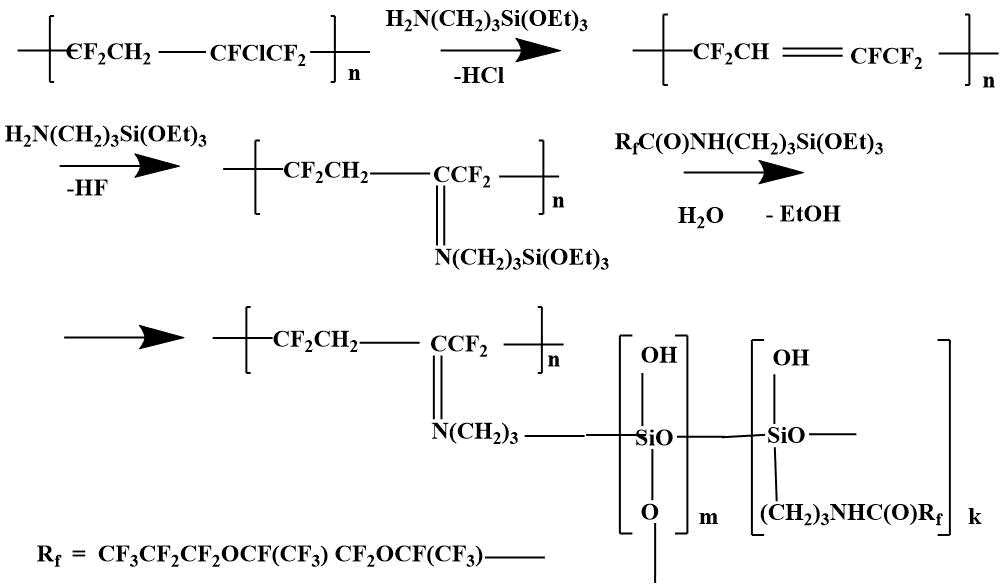
The results of thermo-gravimetric analysis of SKF 32 and F-39 composition on air and in argon are shown in the pic. 3-4: SKF-32: AMEO=45.5: 48.5: 6 (mass.%). The rubber SKF-32 starts to loose mass (degrade) on air at 350 °C, the coke residue at 500 °C is 0%, in argon it starts to degrade at 370 °C, the coke residue in argon at 500 °C = 12 %. The triple composition starts to lose mass at ≈ 300 °C. In argon degradation flows a little faster. In the inert atmosphere the coke residue at 500-800 °C is ≈ 22 %, on air at 500-800 °C – 5-6 %.
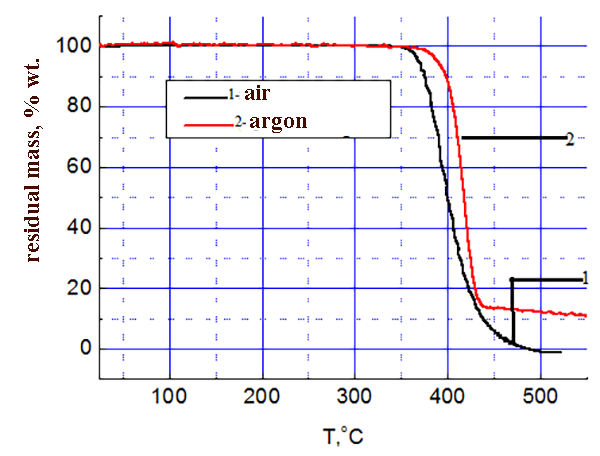
Pic. 3 SKF-32 TGA data.
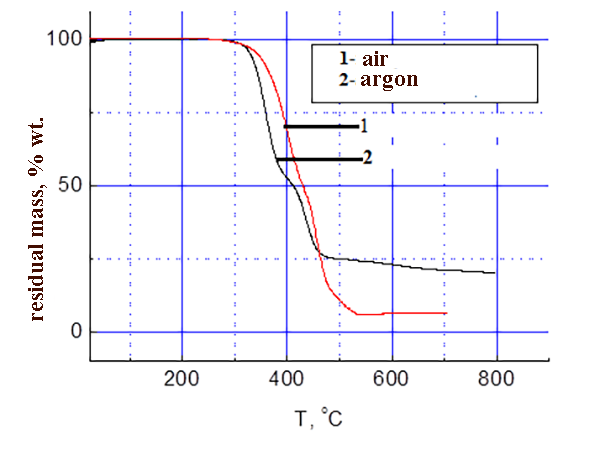
Pic.4 TGA data of composition F-39: SKF-32: AMEO=45.5: 48.5: 6 (mass.%).
Films were prepared from the composition F-39: SKF-32: AMEO=45.5: 48.5: 6(mass. %) and its bursting strength and elongation strength were determined. Composition’s bursting strength (σ) was 2-4 times higher than that of unvulcanized SKF-32; elongation strength (ε) was twice lower. (Table)
Table.
|
Code |
ε, % |
σ, MPa |
|
Composition |
460-600 |
5-6 |
|
SKF-32 |
800-1200 |
1.5-3 |
As a result, compositions were obtained from Fluorosam-39 monomer (triethoxysilylpropylamide perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid), fluororubber SKF-32 (vinylidene fluoride with trifluorochloroethylene co-polymer), in the presence of AMEO(γ- aminopropyltriethoxysilane) and synthetic method was suggested. Fluororubber content in the compositions varied from 10 to 55% (mass.) Thermomechanical properties of the composition, obtained from Fluorosam-39:SKF-32:AMEO at the ratio 45.5: 48.5: 6 (mass.%) were studied. It was found out that glass transition point (-20 °C) of such composition is significantly lower than that of Fluorosam-39 polymer (230 °C). Composition’s bursting strength (σ) was 2-4 times higher than that of unvulcanized SKF-32; elongation strength (ε) was twice lower.
Experimental
Termomechanical tests were held on TMA Q400 (TAInstruments, USA) at static loading, sonde diameter 2.8 mm, F = 100 Н, cooling rate ~ 5 °С/min, heating rate 5 °С/min.
Thermogravimetric tests were held on «Derivatograth-C» (МОМ, Hungary) on air and in argon at heating rate 10 °С/min on samples with mass ~20 мг.
The glass transition temperature was determined using differential scanning calorimetry method on DSC-822e (Mettler Toledo, Switzerland) at heating rate 10 ºС / min on samples with mass ~10 mg.
Films’ mechanical characteristics were determined on Universal Materials Testing Machine LR50KPlus (“Lloyd Instruments”, UK) at a rate of deformation 100 mm/min.
Caoutchouc SKF-32 was given by the «RIAS» company. Mooney viscosity 150-180.
AMEO was given by OJSC «Penta» Moscow. Boiling temp. 79-80 °C /3 mm Hg. 97% purity.
Fluorosam-39 monomer was obtained in IOC RAS laboratory. B.p.=140-144 °C /1 mm Hg, nD20-1,3548.
Film obtainment on the base of Fluorosam-39 monomer in the presence of SKF-32 and AMEO as a catalyst and co-reactant
A polypropylene Petrie dish was sequentially charged with 0.64 g of SKF-32 in the form of 5% solution in ethyl acetate (48.5 mass. %), 0.08 g, AMEO (6 mass. %) and 0.6 g of Fluorosam -39 monomer (45.5 mass %), stirred for 5 minutes. The film was formed by curing of received mixture at room temperature within 3 days and further heat treatment at 60 ̶ 80 °C up to constant mass. As a result an opaque uniform film was obtained.
Authors give thanks to the workers of N.D. Zelinsky Institute of Organic Chemistry Russian Academy of Science M.M. Buzin and G.G. Nikiforova for their help with doing TMA, TGA of obtained polymers.
References
- Ananikov V.P., Galkin K.I., Egorov M.P., Sakharov A.M., Zlotin S.G., Redina E.A., Isaeva V.I., Kustov L.M., Gening M.L, Nifantiev N.E. Challenges in the development of organic and hybrid molecular systems, Mendeleev Commun., 2016, 26, 365-374
- Ananikov V.P., Eremin D.B., Yakukhnov S.A., Dilman A.D., Levin V.V., Egorov M.P., Karlov S.S., Kustov L.M., Tarasov A.L., Greish A.A., Shesterkina A.A., Sakharov A.M., Nysenko Z.N., Sheremetev A.B., Stakheev A.Y., Mashkovsky I.S., Sukhorukov A.Y., Ioffe S.L, Terent’ev A.O., Vil V.A., Tomilov Y.V., Novikov R.A., Zlotin S.G., Kucherenko A.S., Ustyuzhanina N.E., Krylov V.B., Tsvetkov Y.E., Gening M.L., Nifantiev N.E. Organic and hybrid systems: from science to practice, Mendeleev Commun., 2017, 27, 425-438
- Venediktova M.A., Naumov I.S., Chikun A.M., Eliseev O.A. Aviation materials and technologies, 2014. No. S3, P. 17–24 (in Russian).
- Kraev I.D., Popkov O.V., Sorokin A.E., Yurkov., G.Yu. Perspectives of using silicone polymers in the development of advanced materials and coatings for different purposes. The Electronic scientific journal "Proceedings of VIAM". 2017. No. 12 (in Russian).
- Novitskaya S.P., Nudelman Z.N., Dontsov A.A. Fluoroelastomers. Moscow. Chemistry. 1988. P. 4 (in Russian).
- Sakharov A.M., Popovich M.Yu., Krukovsky S.P. Fluorine Notes, 2017, 5 (114)
- Pasiorik K. Fluoropolymers. Mir. 1975. P. 242 (in Russian).
Recommended for publication by Prof. S. M. Igoumnov
Fluorine Notes, 2019, 123, 3-4
