Received: February 2019
Fluorine Notes, 2019, 123, 9-10
1,1-DIFLUORO-2(1H)-NAPHTHALENONES: SYNTHESIS, PROPERTIES AND APPLICATIONS
P. Zaikin, O. Dyan
1NIOC SB RAS, 9 Lavrentiev Ave., Novosibirsk, 630090, Russian Federation
E-mail: zaikin@nioch.nsc.ru
Organofluorine compounds attract much attention in industry, pharmacology and materials design. Such an importance requires the development of effective methods of synthesis of organofluorine compounds based both on the incorporation of fluorine atoms in organic targets and use of fluorine-containing building blocks to construct more complex structures.
Our efforts in the development of effective fluorination methods are based on widely applicable direct electrophilic fluorination [1]. We have recently developed the solvent-free methodology for the fluorination of aromatic compounds with Selectfluor reagent. Combining solvent-free synthesis with vacuum sublimation to obtain products allows us to reach quite a low values of E-factor (3.7-5.7) [2]. The perspectives of using water and aqueous solvents as media for electrophilic fluorination was also demonstrated earlier [3].
We have found that easily obtained products of the electrophilic fluorination of various 2-naphthols – 1,1-difluoro-2(1H)-naphthalenones are promising fluorine-containing building blocks for the synthesis of complex organofluorine molecules. We’ve explored the reactivity of the abovementioned compounds in the Diels-Alder reaction with simple dienes and have investigated the solvent effects in reaction with model diene [4].
Synthetic applicability of 1,1-difluoro-2(1H)-naphthalenones in the Diels-Alder reaction was further demonstrated in the synthesis of condensed tetracyclic polyaromatic products – tetraphenones and substituted tetraphenes [5]. The latter was demonstrated to have relatively low values of HOMO-LUMO gap (3.0 eV) that makes it promising candidates to develop organic semiconductors.
This work was supported by the Russian Foundation for Basic Research, grants 16-33-00944, 18-33-00529.
References
- Zaikin, P.; Borodkin, G. Electrophilic and Oxidative Fluorination of Aromatic Compounds. In Late-Stage Fluorination of Bioactive Molecules and Biologically-Relevant Substrates, Elsevier, 2019, 105.
- Zaikin, P. et al. Eur. J. Org. Chem., 2017, 2017(17), 2469.
- Borodkin, G.; Zaikin, P. Chem. Sustain. Dev., 2011, 19(6), 593.
- Zaikin, P. et al. J. Fluorine Chem., 2017, 199, 20.
- Dyan, O. et al. J. Fluorine Chem., 2018, 210, 88.
Transformation of polyfluoroorganyltrifluoroborates, M[RfBF3], without C-BF3 cleavage
N. Yu. Adonin 1, V.V. Bardin 2
1 G.K. Boreskov Institute of Catalysis of the Siberian Branch of the Russian Academy of Sciences, 5 Acad. Lavrent’ev Ave., 630090 Novosibirsk, Russian Federation
2N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of the Russian Academy of Sciences, 9 Acad. Lavrentiev Ave., 630090 Novosibirsk, Russian Federation
E-mail: bardin@nioch.nsc.ru
Polyfluorinated organyltrifluoroborates can be prepared by three ways: carbon-boron bond formation, re-functionalization at boron RfBX2 to M[RFBF3] or M[RFBY3] to M[RFBF3], and transformation of M[R'FBF3] to M[RFBF3]. Here the latter processes are reviewed.
1. Transformations of polyfluoroalkenyl- and alkynyl moiety in M[RFBF3].
a) Photoinduced cis/trans isomerization:
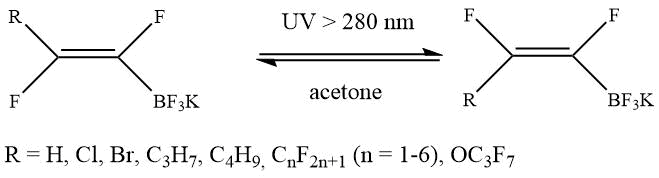
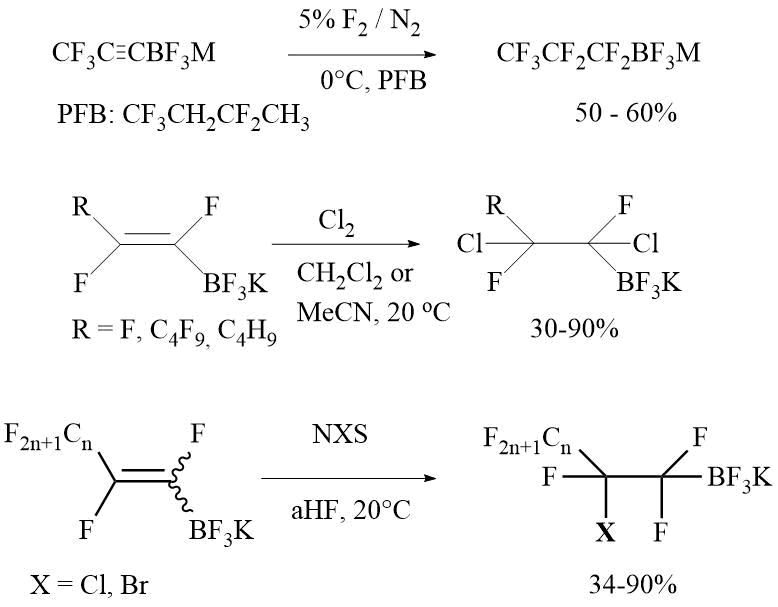
b) Addition of halogene and interhalogene across double or triple carbon-carbon bond:
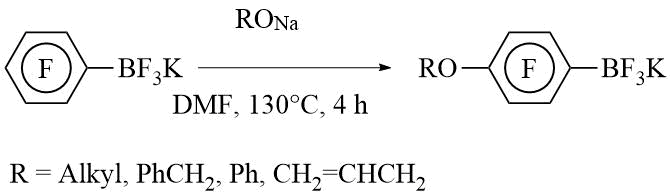
3. Nucleophilic substitution in pentafluorophenyl moiety.
a) With O-nucleophiles:
b) With N-nucleophiles:
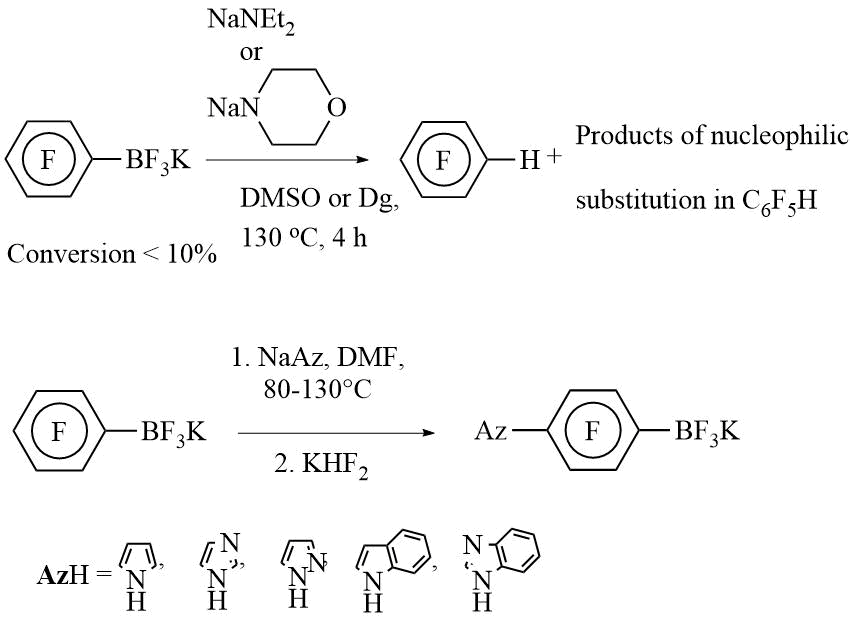
c) With C-nucleophiles:
DAIKIN'S BUSINESS OVERVIEW
Fumihiko Yamaguchi
Technology and Innovation Center DAIKIN INDUSTRIES, LTD., 1-1 Nishi-Hitotsuya Settsu Osaka 566-8585, Japan
E-mail: fumihiko.yamaguchi@daikin.co.jp
1. Introduction to Daikin Industries
Daikin Industries has two businesses, machinery with a focus on air conditioning and chemicals. This time, I introduce the Daikin's history, business introduction and business contents of the chemistry division.
2. Attractiveness and Applications of Fluorinated Materials
The history of the development and industrialization of fluorinated resins, which began with the discovery
of DuPont in 1938 and Planckett's PTFE(Polytetrafluoroethylene), is similar to that of other engineering
plastics, but it is being actively developed for a variety of applications, and the industrialization
of new materials continues. This is because fluorinated materials have many unique functions, and
fluorinated materials have been attracting attention as an indispensable material in a variety of
fields. In particular, it plays an important role in industry and in our daily lives in a variety
of fields, including materials for semiconductor processes, materials for automobiles, and textile
processing agents that take advantage of the properties of heat resistance, chemical resistance,
weather resistance, non-adhesion, and water repellency.
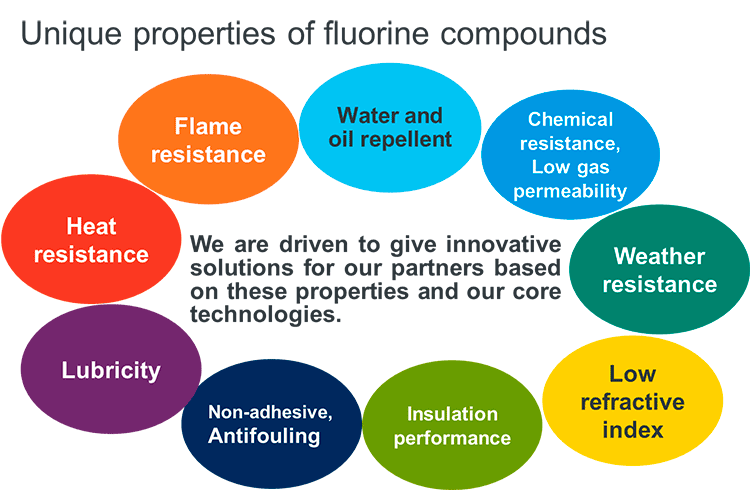
Examples of product development in these fields and synthetic intermediates derived from TFE monomers are presented.
Selective one-pot mono-, di-, and triaminodefluorination of polyfluoroarenes in anhydrous ammonia – the convenient and shortest route to monomers and building blocks for fluorinated polyimides and other hi-tech materials
T.A. Vaganova, E.V. Malykhin
N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of the Russian Academy of Sciences, 9 Acad. Lavrentiev Ave., 630090 Novosibirsk, Russian Federation
E-mail: malykhin@nioch.nsc.ru
A technologically advanced and ecofriendly method of aminodefluorination of polyfluorinated benzenes
[1a], naphthalenes [1b], and pyridines [1c] has been elaborated. The reaction proceeds effectively
in the anhydrous liquid ammonia as the reagent and a solvent simultaneously; product yields achieve
85-95%. The specific temperature of the process allows a certain amount of amino groups to be selectively
introduced. Products of the reactions, i.e. high purity polyfluoroaromatic mono- and diamines serves
as building blocks and monomers in the synthesis of drugs, agricultural and veterinary formulations,
polyfluoroaromatic polyimides, and molecular co-crystals.
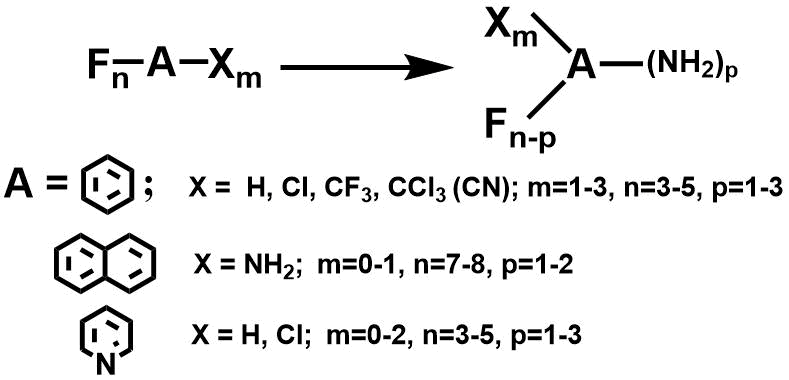
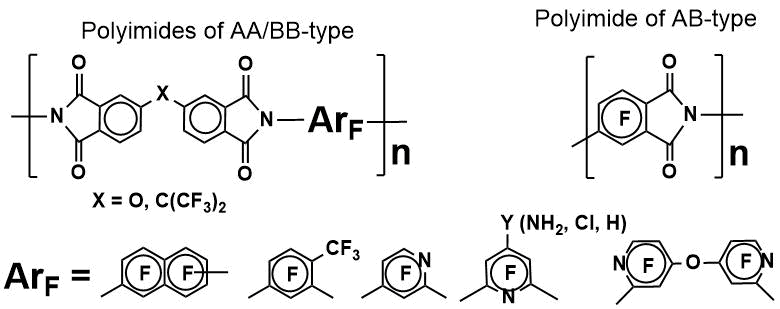
Highly fluorinated aromatic polyimides have excellent optical performance (high transparency and low optical transmission losses in the near-IR and UV-vis regions), thermal stability (high glass transition and weight loss temperatures), good dielectric properties and high hydrophobicity. These characteristics determine their use in modern optoelectronic applications. We have developed technological methods for the preparation of highly fluorinated polyimides [2a], which became the basis for obtaining hi-tech materials, for example, transparent and thermostable matrixes for NLO materials [2b], modifiers-hardeners for epoxy insulating coatings [2c].
Organic cocrystals are a hot trend in the design of materials with nonlinear optical, photochromic, magnetic and electroactive properties. Solid-state transitions in a crystal under external action are used in thermal and photochromic molecular sensors, switches and molecular machines. Co-crystals serve as molecular containers for regio-and stereoselective reactions, as delivery systems for pharmaceutical ingredients in vivo, etc. We have synthesized and investigated the properties of the new objects for crystal engineering – associates of polyfluoroaromatic mono- and diamines and macrocyclic ethers, which form supramolecular hydrogen-bound assembles [3a].
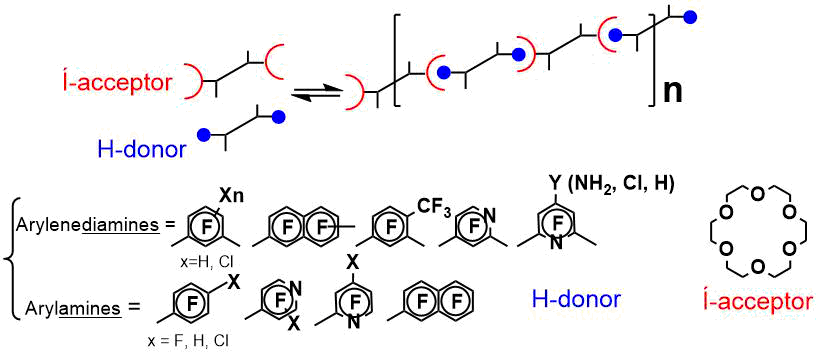
For a number of polyfluoroaromatic amines, there are found significant changes in the fluorescence characteristics due to the formation of associates with 18-crown-6 – build-up or quenching of the emission [3b]. These phenomena seems to be promising for using the organic cocrystals to make solid-state supramolecular chemospecific indicators and sensors.
References
- a) T.A. Vaganova, S.Z. Kusov, V.I. Rodionov, I.K. Shundrina, E.V. Malykhin, Russ. Chem. Bull. Int. Ed. 2007, 56, 2239–2246; V.I. Rodionov, T.A. Vaganova, E.V. Malykhin, J. Fluorine Chem. 2015, 180, 98–102; T.A. Vaganova, V.I. Rodionov, I.P. Chuikov, E.A. Chochrina, E.V. Malykhin, J. Fluorine Chem. 2017, 200, 84–90; b) T.A. Vaganova, S.Z. Kusov, V.I. Rodionov, I.K. Shundrina, G.E. Sal’nikov, V.I. Mamatyuk, E.V. Malykhin, J. Fluorine Chem. 2008, 129, 253–260; c) S.Z. Kusov, V.I. Rodionov, T.A. Vaganova, I.K. Shundrina, E.V. Malykhin, J.Fluorine Chem. 2009, 130, 461–465.
- a) I.K. Shundrina, T.A. Vaganova, S.Z. Kusov, V.I. Rodionov, E.V. Karpova, E.V. Malykhin, J. Fluorine Chem. 2009, 130, 733–741; I.K. Shundrina, T.A. Vaganova, S.Z. Kusov, V.I. Rodionov, E.V. Karpova, E.V. Malykhin, J. Fluorine Chem. 2011, 132, 207–215; T. Vaganova, I. Shundrina, S. Kusov, E. Karpova, I. Bagryanskaya, E.V. Malykhin, J. Fluorine Chem. 2012, 135,129–136; T.A. Vaganova, I.K. Shundrina, S.Z. Kusov, V.I. Rodionov, E.V. Malykhin, J. Fluorine Chem. 2013, 149, 57–64; b) T.A. Vaganova, A.I. Plekhanov, A.E. Simanchuk, S.L. Mikerin, E.V. Spesivtsev, E.V. Karpova, T.S. Frolova, E.V. Malykhin, J. Fluorine Chem. 2017, 195, 70–78; c) T.A. Vaganova, T.A. Brusentseva, A.A. Filippov, E.V. Malykhin, J. Polym. Res. 2014, 11, 21:588.
- a) S.Z. Kusov, Yu.V. Gatilov, I.Yu. Bagryanskaya, G.V. Romanenko, T.A. Vaganova, I.K. Shundrina, E.V. Malykhin, Russ. Chem. Bull. Int. Ed., 2010, 59, 382–390; T.A. Vaganova, S.Z. Kusov, I.K. Shundrina, Yu.V. Gatilov, E.V. Malykhin, J. Mol. Struct. 2011, 109–115; T.A. Vaganova, S.Z. Kusov, I.K. Shundrina, Yu. V. Gatilov, E.V. Malykhin, J. Mol. Struct. 2013, 1033, 27–37; T.A. Vaganova, Yu.V. Gatilov, E.V. Malykhin, Russ. Chem. Bull. Int. Ed., 2015, 64, 1746–1756; T.A. Vaganova, Yu.V. Gatilov, V.I. Rodionov, E.V. Malykhin, J. Struct. Chem. 2016, 56, 1225–1234; T.A. Vaganova, Yu.V. Gatilov, S.E. Malykhin, D.P. Pishchur, Yu.V. Larichev, V.I. Rodionov, E.V. Malykhin, J. Mol. Struct., 2017, 1133, 122–134; b) T.A. Vaganova, Yu.V. Gatilov, D.P. Pishchur, I.P. Chuikov, E.V. Malykhin, CrystEngComm, 2018, 20, 807-817.
Company Overview and Introduction of Some Fluoroorganic Materials in AGC
Yoshitomo Morizawa
Innovative Technology Research Center, Technology General Division, AGC Inc., 221-8755, JAPAN, Yokohama-shi, 1150, Hazawa-cho, Kanagawa-ku,
E-mail: Yoshitomi-morizawa@agc.com
AGC Inc. manufactures a variety of organofluorine products, which have been widely used in the industry as thermoplastics, elastomers, membranes, textile finishes, coatings, and functional materials, including pharmaceutical and agricultural agents, based on their unique combinations of properties [1].
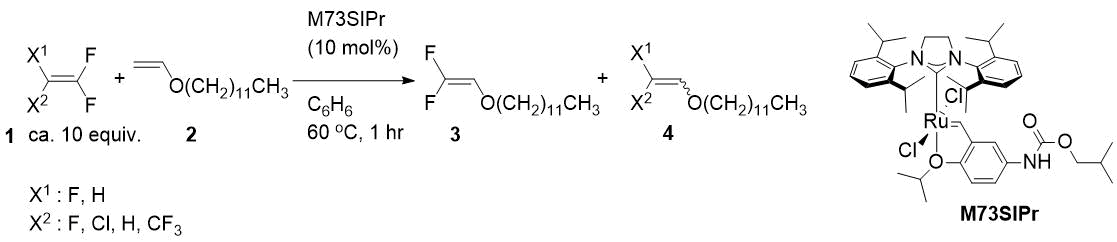
Hydrogen fluoride, fluorine gas, and fluoro monomers as a fluorine source or a fluorinated building block are important raw materials in fluoro chemical industry. Here, first, unique fluorination method to produce perfluorinated compounds with fluorine gas, starting from hydrocarbon precursors is introduced. The method is applicable to a wide range of organic compounds and has a great advantage for synthesis of fluorine-containing molecules at will, simply by construction of the corresponding hydrocarbon skeleton [2].
Then, a successful ruthenium-catalyzed olefin cross-metathesis with fluoroolefins is demonstrated, although this has been considered to be difficult to perform by both theoretical and experimental precedents. Tetrafluoroethylene and the related fluoroolefins such as trifluoroethylene, chlorotrifluoroethylene, and hexafluoropropylene are applicable to this transformation reaction [3].
References
- http://www.agc-chemicals.com/na/en/index.html
- T. Okazoe, J. Fluorine Chem., 2015, 174, 120-131
- Y. Takahira, Y. Morizawa, J. Am. Chem. Soc., 2015, 137, 7031–7034.
Synthesis of SF5-Pyridines
Norio Shibata
Department of Nanopharmaceutical Sciences & Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555, Japan
E-mail: nozshiba@nitech.ac.jp
Chemistry of SF5-substituted compounds has gained attention due to the unique property of SF5-group. Recent substantial efforts in synthetic SF5 chemistry have made simple SF5 aromatics easily available. However, the preparation of SF5 pyridines remains a challenge (Fig. 1).
Unlike SF5-benzenes, which can be prepared at an industrial scale by direct fluorination of aryl disulfides or by Umemoto’s procedure by the oxidative chloro-tetrafluorination of aryl disulfides to arene-sulfur chlorotetrafluorides (SF4Cl-arenes) followed by a chloride/fluoride exchange reaction with fluoride, heteroaromatic systems were difficult to follow this pathway and different strategies are used. In 2015, Dolbier and co-workers reported the first general method for the synthesis of ortho-SF5-substituted pyridines using Umemoto’s method in which 2,2’-dipyridyl disulfides interacted with the KF/Cl2/MeCN system to afford SF4Cl pyridines. For further transformation of these sulfur chlorotetrafluorides to SF5 pyridines, silver fluoride (AgF) was found to be the principal reagent. Despite this, m- and p-pyridine disulfides failed to form SF4Cl-pyridines under same conditions. Herein we disclose a general method for the preparation of m- and p-SF5-pyridines for the first time [1]. First, at least one fluorine atom in the pyridine ring effectively reduces the basicity of nitrogen, inhibiting it from entering a decomposition pathway. Second, fluorine induces greater stability of the SF4Cl moiety. Moreover, the C-F bond at the ortho-position of pyridine can be easily activated towards nucleophilic aromatic substitutions under suitable conditions selected.
References
- (a) M. Kosobokov, B. Cui, A. Balia, K. Matsuzaki, E. Tokunaga, N. Saito, N. Shibata, N. Angew. Chem. Int. Ed. 2016, 55, 10781;
(b) P. Das, M. Takada, K. Matsuzaki, N. Saito, N. Shibata, Chem. Commun. 2017, 53, 3850;
(c) B. Cui, M. Kosobokov, K. Matsuzaki, E. Tokunaga, N. Shibata, Chem. Commun. 2017, 53, 5997;
(d) B. Cui, S. Jia, E. Tokunaga, N. Saito, N. Shibata, Chem. Commun. 2017, 53, 12738;
(d) P. Das, E. Tokunaga, N. Shibata, Tetrahedron Lett. 2017, 58, 4803.
A new approach to fluorinated nitronyl nitroxides
Evgeny Tretyakov,1,2 Pavel Fedyushin,1,2 Borislav Koshcheev,1 Alexander Maksimov,1 Elena Panteleeva,1,2 Irina Bagryanskaya,1,2 Tatyana Rybalova,1,2 Elena Zaytseva,1,2 Elena Bagryanskaya1,2
1N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of the Russian Academy of Sciences, 9 Acad. Lavrentiev Ave., 630090 Novosibirsk, Russian Federation
2Novosibirsk State University, 2 Pirogova Str., Novosibirsk 630090, Russian Federation
E-mail: tretyakov@nioch.nsc.ru
Using nitroxide radicals to construct molecular magnets, spin labels, redox active materials, etc. stimulates
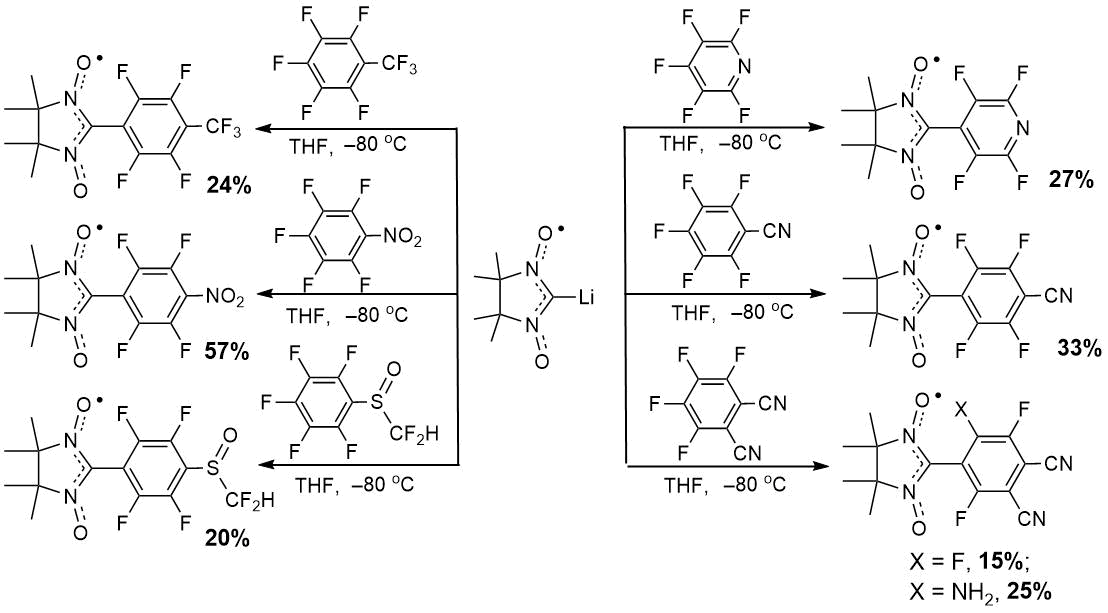
the development of ways of their preparing. Recently it was shown that 4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl
lithium derivative (Li–NN) reacted with perfluorobenzonitrile to form 2-(4-cyanotetrafluorophenyl)-substituted
nitronyl nitroxide [1]. To explore this approach, reactions of Li–NN with different polyfluoroarenes
were investigated. As the result, new multifunctional fluorinated nitronyl nitroxides were prepared
and completely characterized [2].
The proposed strategy can be useful for planning of the retrosynthesis of nitroxides bearing strong electron-withdrawing groups. Such paramagnetics arouse much interest in the field of molecular design of magnets; they can be used for creation of paramagnetic chemical sensors and organic rechargeable batteries.
References
- E. V. Tretyakov, P. A. Fedyushin, E. V. Panteleeva, D. V. Stass, I. Yu. Bagryanskaya, I. V. Beregovaya, A. S. Bogomyakov. J. Org. Chem. 2017, 82, 4179−4185.
- P. Fedyushin, E. Panteleeva, I. Bagryanskaya, K. Maryunina, K. Inoue, D. Stass, E. Tretyakov. J. Fluor. Chem., 2019, 217, 1–7.
Acknowledgements
This work was supported by the RFBR (project 18-33-00203\18).
Interesting Isomerization Reactions Initiated by the Abstraction of a Proton Activated by a CF3 group
Takashi Yamazaki1
1Division of Applied Chemistry, Institute of Engineering, Tokyo University of Agriculture and Technology, 2-24-16 Nakamachi, Koganei 184-8588, Japan
E-mail: tyamazak@cc.tuat.ac.jp
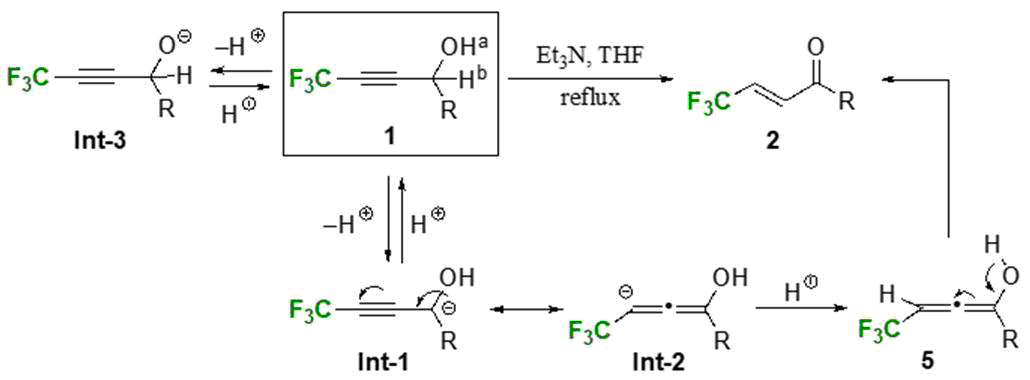
Smooth isomerization of 4,4,4-trifluorinated propargylic alcohols 1 opened a new and convenient route to readily get access to the corresponding α,β-unsaturated ketones 2 just by heating its THF solution at the reflux temperature in the presence of Et3N.[1] This reaction was considered to be initiated by the abstraction of Hb in 1 and reprotonation occurred at the anionic site in Int-2 to give 5 whose keto-enol isomerization afforded the desired products 2 in good to excellent yields. However, we at first thought that the deprotonation of Ha would be the main path. Our computation on these transformations clarified that, contrary to our initial surmise, Hb was more acidic than Ha on the basis of the energetic difference between Int-1 and Int-3 (ΔE=1.048 kcal/mol when R=Me) and this difference became bigger when the R group was Ph (ΔE=7.869 kcal/mol when R=Ph). It is quite apparent that a CF3 group plays a key role here because Int-3 was found to be preferred by 7.803 kcal/mol when R=Ph and a CF3 group was substituted for a CH3 moiety.
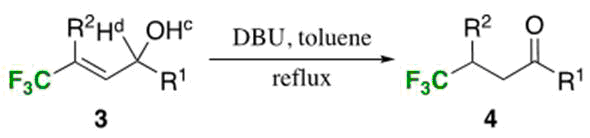
Our computation also anticipated the similar isomerization starting from the allylic alcohols 3 due to a slight carbanion preference (0.321 kcal/mol when R=Ph) and this was experimentally the case, and 3 easily isomerized to 4 under the action of such a stronger amine base as DBU at toluene reflux. Utilization of optically active substrates (R1=Ph; R2=Ph, 4-MeOC6H4-, 4-FC6H4-) recorded a high stereoselectivity with up to >99% chirality transmission.
References
- T. Yamazaki, T. Kawasaki-Takasuka, A. Furuta, and S. Sakamoto, Tetrahedron 2009, 65, 5945–5948.
- Y. Hamada, T. Kawasaki-Takasuka, and T. Yamazaki, Beilstein J. Org. Chem. 2017, 13, 1507–1512.
1,1-DIFLUORO-2(1H)-NAPHTHALENONES: SYNTHESIS, PROPERTIES AND APPLICATIONS
P. Zaikin, O. Dyan
1N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of the Russian Academy of Sciences, 9 Acad. Lavrentiev Ave., 630090 Novosibirsk, Russian Federation
E-mail: zaikin@nioch.nsc.ru
Organofluorine compounds attract much attention in industry, pharmacology and materials design. Such an importance requires the development of effective methods of synthesis of organofluorine compounds based both on the incorporation of fluorine atoms in organic targets and use of fluorine-containing building blocks to construct more complex structures.
Our efforts in the development of effective fluorination methods are based on widely applicable direct electrophilic fluorination [1]. We have recently developed the solvent-free methodology for the fluorination of aromatic compounds with Selectfluor reagent. Combining solvent-free synthesis with vacuum sublimation to obtain products allows us to reach quite a low values of E-factor (3.7-5.7) [2]. The perspectives of using water and aqueous solvents as media for electrophilic fluorination was also demonstrated earlier [3].
We have found that easily obtained products of the electrophilic fluorination of various 2-naphthols – 1,1-difluoro-2(1H)-naphthalenones are promising fluorine-containing building blocks for the synthesis of complex organofluorine molecules. We’ve explored the reactivity of the abovementioned compounds in the Diels-Alder reaction with simple dienes and have investigated the solvent effects in reaction with model diene [4].
Synthetic applicability of 1,1-difluoro-2(1H)-naphthalenones in the Diels-Alder reaction was further demonstrated in the synthesis of condensed tetracyclic polyaromatic products – tetraphenones and substituted tetraphenes [5]. The latter was demonstrated to have relatively low values of HOMO-LUMO gap (3.0 eV) that makes it promising candidates to develop organic semiconductors.
References
- Zaikin, P.; Borodkin, G. Electrophilic and Oxidative Fluorination of Aromatic Compounds. In Late-Stage Fluorination of Bioactive Molecules and Biologically-Relevant Substrates, Elsevier, 2019, 105.
- Zaikin, P. et al. Eur. J. Org. Chem., 2017, 2017(17), 2469.
- Borodkin, G.; Zaikin, P. Chem. Sustain. Dev., 2011, 19(6), 593.
- Zaikin, P. et al. J. Fluorine Chem., 2017, 199, 20.
- Dyan, O. et al. J. Fluorine Chem., 2018, 210, 88.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research,grants 16-33-00944, 18-33-00529
Carbonylation of perfluorobenzocycloalkenes and their perfluoroalkyland pentafluorophenyl derivatives in SbF5 medium
Ya. V. Zonov1,2, V. M. Karpov1, T.V. Mezhenkova1, V. E. Platonov1
1N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of the Russian Academy of Sciences, 9 Acad. Lavrentiev Ave., 630090 Novosibirsk, Russian Federation
2Novosibirsk State University, 2 Pirogova Str., Novosibirsk 630090, Russian Federation
E-mail: yzonov@nioch.nsc.ru
We have found [1-3] that perfluorobenzocycloalkenes and a number of their perfluoroalkyl and pentafluorophenyl derivatives undergo carbonylation under the action of CO in the presence of SbF5 and it is the first example of carbonylation of perfluorinated organic compounds. Most of these transformations readily proceed at room temperature and atmospheric pressure with complete convertion of starting compound, addition of more than one CO molecule occurs in a number of cases.
Perfluoroinated benzocyclobutene derivatives in the CO–SbF5 system undergo carbonylation/four-membered ring opening (or expansion) tandem reaction. Thus, perfluorobenzocyclobutenes (1) with unsubstituted aliphatic cycle adds two CO molecules to form isochromenes (2). Perfluoro-1-alkylbenzocyclobutenes (3) undergo monocarbonylation at the substituted position of the four-membered ring to form 2-(2-methylphenyl)alkenoic acid derivatives (4) [1].
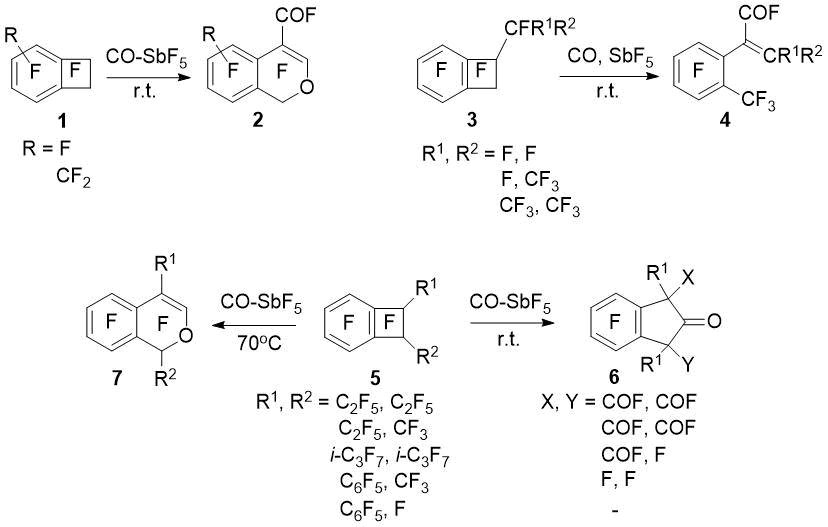
1,2-Disubstituted perfluorobenzocyclobutenes (5) in the reaction with CO–SbF5 at room temperature give indan-2-one derivatives (6) with or without fluorocarbonyl groups at the benzylic positions. Increase in the reaction temperature up to 70оС leads to isochromenes (7) formations [2]. Carbonylation of perfluoro-1-phenylbenzocyclobutene also gives perfluoro-4-phenylsochromene [1].
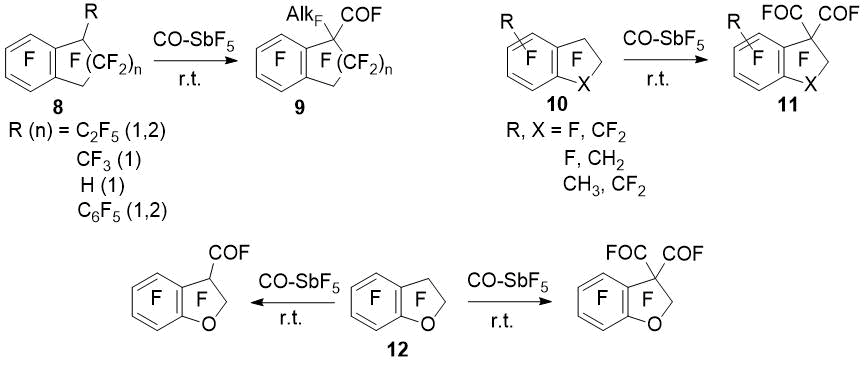
Polyfluoroinated indan and tetralin derivatives in the CO–SbF5 system undergo carbonylation without transformation of the aliphatic ring. The degree of conversion varies in wide range and can reaches 100%. Thus, monosubstituted derivatives (8) of perfluorinated indan and tetralin undergo monocarbonylation at the substituted position of the aliphatic ring to give acyl fluorides (9). Polyfluoroindans (10) without the CFX fragment at the benzylic position give products of dicarbonylation to form indan-1,1-dicarbonyl difluorides (11). For perfluoro-2,3-dihydrobenzofuran (12), formation of both mono- and dicarbonylation products are possible depending on amount of antimony pentafluoride [3].
References
- Ya.V. Zonov, V.M. Karpov, V.E. Platonov, J. Fluorine Chem. 2014, 162, 71–77.
- Ya.V. Zonov, V.M. Karpov, T.V. Mezhenkova, T.V. Rybalova, Yu.V. Gatilov, V.E. Platonov, J. Fluorine Chem. 2016, 188, 117–125.
- Ya.V. Zonov, V.M. Karpov, T.V. Mezhenkova, V.E. Platonov, J. Fluorine Chem. 2018, 214, 24–34.
Fluorine Notes, 2019, 123, 9-10
