Received: May 2019
DOI 10.17677/fn20714807.2019.03.01
Fluorine Notes, 2019, 124, 1-2
The investigation of the polymerization of 2,3,4,5,6-pentafluorostyrene in the presence of 2-cyano-2-propyl-dithiobenzoate by 1H-NMR spectroscopy
K.E.Chekurov*, A.I. Barabanova, A.S. Peregudov, A.R. Khokhlov
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov St. 28, GSP-1, Moscow, Russian Federation
*kirillswim@rambler.ru
Abstract: The radical polymerization of 2,3,4,5,6-pentafluorostyrene (PFS) in the presence of 2-cyano-2-propyl-dithiobenzoate (CPDB) at 50 °C in DMF-d7 was investigated in situ using 1H-NMR spectroscopy. It was established that polymerization occurred with an induction period, which can be due to the formation of a product of selective addition of CPDB and single PFS even before the propagation reaction. For the first time, the chain transfer constant Ctr of CPDB in the polymerization of PFS was estimated. The resulting value of Ctr (Ctr = 77.4±2.2) indicates that CPDB is effective chain transfer agent at polymerization of PFS.
Keywords: 2,3,4,5,6-pentafluorostyrene, amphiphilic diblock copolymers, 1H-NMR spectroscopy.
Introduction
Radical polymerization with reversible chain transfer by the addition-fragmentation mechanism (RAFT polymerization) is one of the methods of controlled synthesis of narrow-dispersed (co)polymers of 2,3,4,5,6-pentafluorostyrene (PFS) with a given structure and MM [1-6]. For the polymerization of PFS, according to the mechanism of RAFT polymerization, so far only four chain transfer agents (CTA) are known: 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid (DDMAT) [1,2], 2-cyano-2-propyl dithiobenzoate (CPDB) [3,4], 4-cyano-4-(thiobenzoylthio)pentanoic acid (CPDTB) and 2-cyano-2-propyl-dithiocarbamate (CPDC) [5,6], and for none of them are effective transfer constants, Ctr, characterizing the activity of CTAs [7,8], not defined.
In the present work, the polymerization of PFS in the presence of CPDB was studied in situ using 1H-NMR spectroscopy, and based on the measurement of current concentrations of PFS and CPDB at the early stages of the process, the transfer constant Ctr of the CPDB was determined.
Experimental part
PFS (P&M-Invest, Russia) were distilled under vacuum before use (P = 0.1 mbar, T = 63 °C). The initiator α-bis-isobutyric acid dinitrile (AIBN) (98%, Sigma-Aldrich, Germany) was twice recrystallized from methanol and dried in vacuum to constant weight. CPDB (> 97%, Sigma-Aldrich, Germany) was used as CTA without prior purification. The solvents were purified according to conventional techniques.
1H NMR spectra were recorded using Avance TM 600 spectrometer by Bruker (Germany) at operating frequency of 600.22 MHz. Chemical shifts were determined relative to the residual signal of CHCl3 in the deuterium solvent (7.28 ppm) and were converted to TMS. Chemical shifts were determined to accuracy no less than 0.001 ppm.
The polymerization of PFS at [PFS] = 2 mol / l, [CPDB] = 5.3 × 10-3 mol / l and [AIBN] = 2.7 × 10-3 mol / l in DMF-d7 at 50 °C was carried out in NMR-tubes intended for recording NMR spectra in vacuum, directly in an Avance TM 600 spectrometer. Before polymerization, the reaction mixture was deaerated by repeated freeze-thaw cycles in vacuum four times. The 1H NMR spectra of the reaction monomer-polymer mixtures were recorded at certain intervals up to the conversion of PFS q = 30.4%. The concentration of PFS and CPDB in the reaction mixture during the reaction was calculated relative to the total integrated intensity of all protons of the reaction mixture. The accuracy of the measurement of the integral intensity was ~ 5%. The proposed approach makes it possible to determine the conversion of CPDB (qCPDB) and PFS (q) at low conversions without PPFS isolating from the reaction mixture.
Results and disscussion
RAFT polymerization of PFS along with the elementary reactions of classical radical polymerization (initiation, propagation and chain termination) includes stages that provide controlled synthesis of poly-2,3,4,5,6-pentafluorostyrene (PPFS): 1) attachment of a growing polymer radical to a low molecular weight CTA with subsequent fragmentation of the intermediate (Int 1), leading to the formation of a macro-CTA and the release of the radical of the leaving group of CTA (Scheme 1, reaction I); 2) reinitiation of the polymer chain by the radical of the leaving group of CTA (Scheme 1, reaction II) and 3) attachment of the growing polymer radical to macro-CTA (Scheme 1, reaction III).
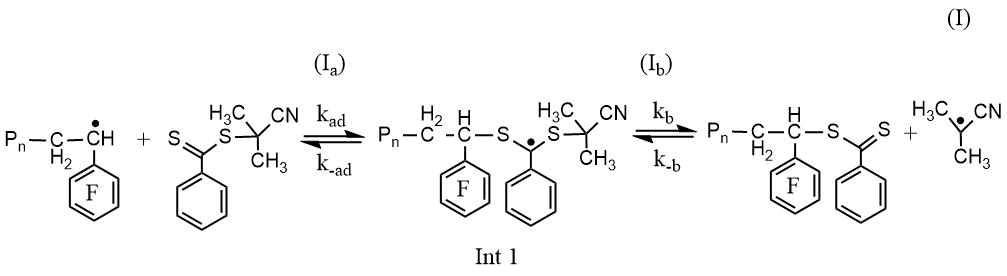
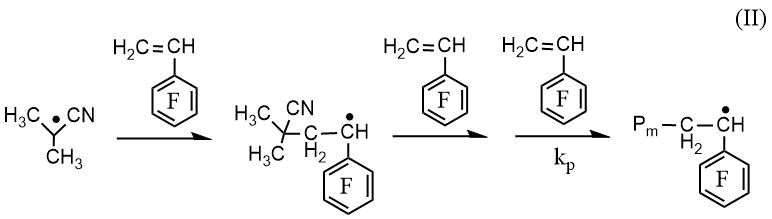
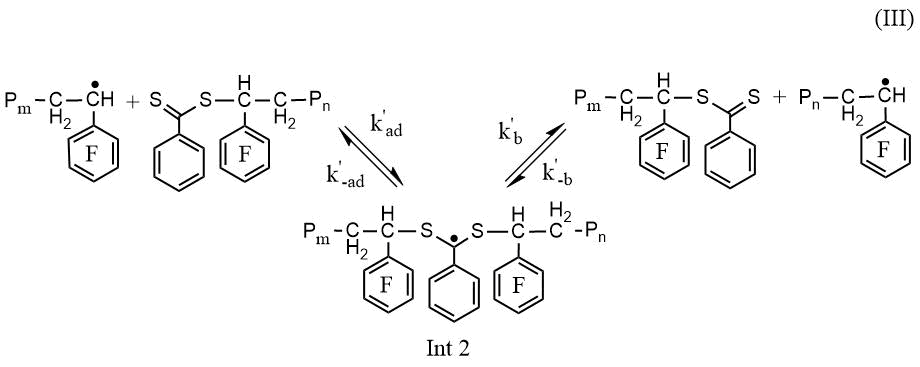
Scheme 1. Scheme of the reactions of attaching a growing PPFS radical to CPDB (I), reinitiationg the chain (II) and attaching a growing PPFS radical to the PPFS CTA (III)
A quantitative measure of the effectiveness of CTA is the chain transfer constant, Ctr, determined by the ratio of the rates of two possible processes of decomposition of the Int1 intermediate (reactions Ia and Ib) (Scheme 1) [7,8]:
C_tr= k_tr/k_p (1)
where ktr and kp are the rate constants of the transfer reaction and chain propagation, respectively.
To evaluate the Ctr in this work, we used the equation:
C_tr≈(dln[CPDB])/(dln[PFS]), (2)
where [PFS] and [CPDB] are current concentrations of PFS and CPBD, respectively [8].
Equation (2) is suitable for determining Ctr when using active CTA's, in the presence of which narrow-dispersed polymers are formed, and the increase in molecular weight of polymers begins already at very low conversion, i.e. under conditions when the conversion of monomer and CTA cannot be considered insignificant [8].
To evaluate the Ctr at CPDB, radical polymerization of the PFS in the presence of the CPDB was investigated at the earliest stages of the process. The current monomer and CTA concentrations were determined without prior isolation of PPFS from the reaction mixture using 1H NMR spectroscopy, recording the 1H NMR spectra of the reaction mixture directly during polymerization at different time intervals and estimating the amount of unreacted PFS and CPDB. We used such approach earlier to determine the composition of copolymers of acrylamide with anion- and cation-active comonomers at different conversions [9-11].
Fig. 1 shows the 1H NMR spectra of the CPDB (Fig 1, 1) and PPFS (Fig. 1, 2). In the CPDB
spectrum, a signal is observed at 1.97 ppm, corresponding to six protons of N-methyl groups (Fig.
1, 1). The signals of the protons of the phenyl group appear as a doublet at 7.94 ppm (two ortho-protons),
a pseudo triplet at 7.42 ppm. (2 meta-protons) and a triplet at 7.59 ppm (1 para-proton).
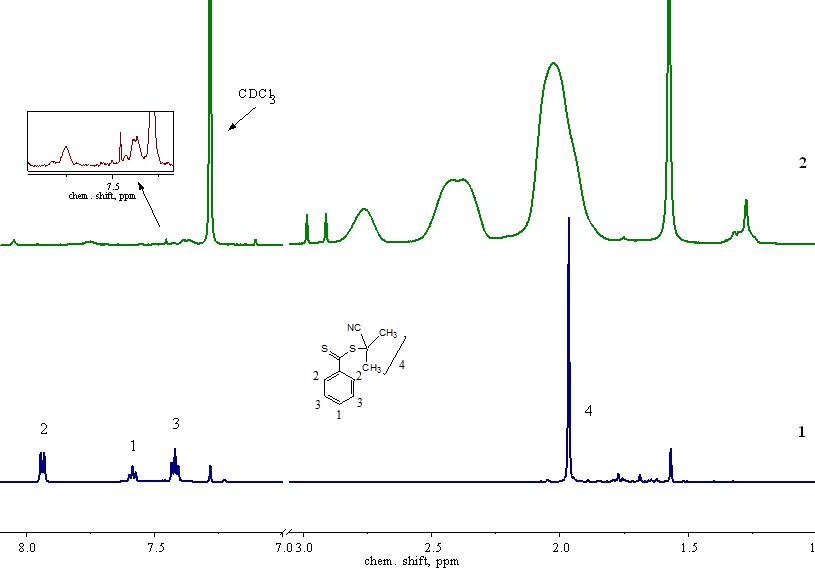
Fig. 1. 1H NMR spectra of CPDB (1) and PPFS, prepared by RAFT polymerization (2), in CDCl3.
In 1H NMR spectrum of PPFS (Fig. 1, 2), broadened signals of five phenyl protons of the stabilizing group of CPDB appear at 7.35 and 7.75 ppm. The signal at 1.97 ppm, referring to the six methyl protons of the –СH3 of the CTA leaving group, in the 1H NMR spectrum of PPFS (Fig. 1, 2), is overlapped by the signal of the –СН2 groups of the main polymer chain in the PPFS and is therefore not separately detected. Signals at 2.05, 2.35 and 2.8 ppm refer to the protons –CH– and –CH2 – groups of the main polymer chain in PPFS (Fig. 1, 2).
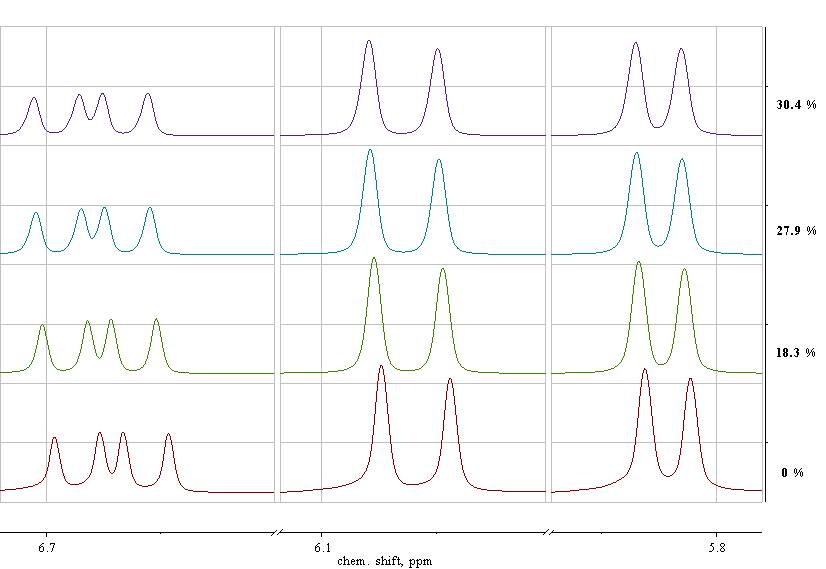
(а)
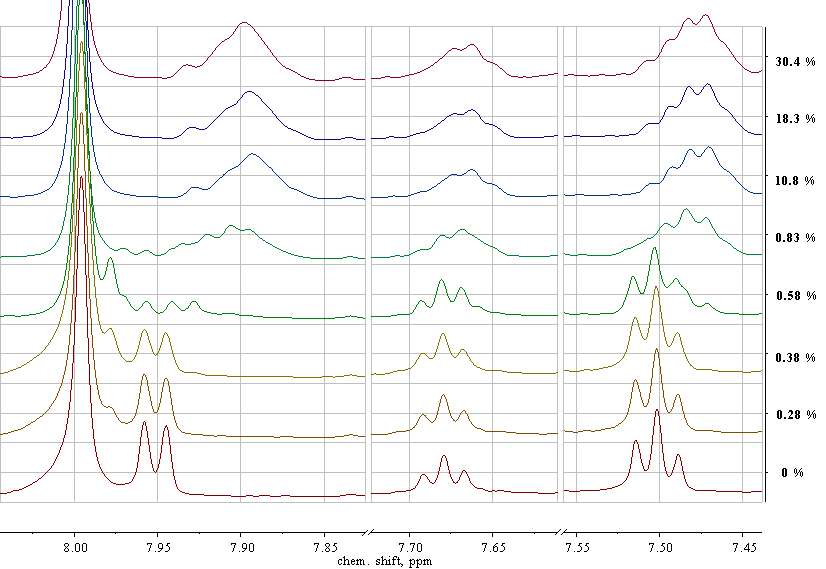
(b)
Fig. 2. Enlarged fragments of the 1H-NMR spectra of the reaction monomer-polymer mixtures before (q = 0%) and after polymerization of PFS at q = 18.3, 27.9 and 30.4% in the chemical shifts ranged from 6.71 to 5.79 ppm (a) and from 8.04 to 7.44 ppm (b). [PFS] = 2 mol / l, [AIBN] = 2.7 × 10-3 mol / l, [CPDB] = 5.3 × 10-3 mol / l, 50 ° С, DMF-d7.
Fig. 2 (a, b) shows fragments of the 1H NMR spectra of monomer-polymer reaction mixtures formed during polymerization at different PFS conversions. In the spectrum of the initial reaction mixture, at q = 0%, six signals of protons of the PFS (Fig. 2, a) and CPDB (Fig. 2, b) are observed. It is important that when recording spectra in DMF-d7, the signals do not overlap.
In the 1H NMR spectrum of PFS in DMF-d7 at q = 0% (Fig. 2, a) there are signals with chemical shifts of 6.90 - 5.75 ppm, related to the three protons of the vinyl group (CH=, CH2=) PFS. The integrated intensity of the signals is proportional to the concentration of PFS in the monomer-polymer mixture. To calculate the amount of unreacted PFS and q, we can use the integrated intensities of each of the signals CH2= and CH=, however, to increase the accuracy, we used their sum.
Resonances in the region of the weak field of the spectrum, which characterizes the monomer mixture at q = 0% (Fig. 2, b), belong to CPDB protons: a doublet at 7.96 and 7.94 ppm, a triplet at 7.69, 7.68 and 7.67 ppm and pseudo triplet at 7.51, 7.50 and 7.49 ppm. It can be seen that in the early stages of the process (q = 0.28, 0.38 and 0.58%) the intensity of the doublet decreases, while the intensity of the triplet and pseudo triplet signals, as well as their position, does not vary, although some signal broadening occurs. Simultaneously with the decrease in the intensity of the doublet, two new signals appear: one at 7.98 ppm, and the second at 7.93 ppm, and with increasing PFS conversion, the intensity of these signals first increases, and starting from q = 0.58%, decreases. When the conversion of the PFS reaches 0.7% all signals range from 7.98 to 7.93 ppm completely disappear and broad signal appears at 7.89 ppm. With a further increase in the conversion of the PFS from 0.83% to 30.4% (Fig. 2, b), there is a slight increase in the intensity of the signals of all protons of the phenyl group. It can be assumed that the signals at 7.98 and 7.93 ppm characterize the ortho-protons of the phenyl group of the product of the selective addition of the initial CPDB to the PFS (C(S)C5H5S[CH2CHC6F5]nC(CH3)2CN), which is formed at the very beginning of the polymerization in the interval q from 0.18 to 0.83% [12 ]. After complete consumption of the CTA, polymerization of the PFS begins, as evidenced by the appearance of wide signal at 7.89 ppm, the intensity of which increases with conversion. The formation of such adducts may be the cause of the induction period during the RAFT polymerization [12].
The concentration of unreacted CPDB was calculated using the integral intensity of the ortho-proton doublet of the CPDB phenyl group with chemical shift at 7.96 and 7.94 ppm. Due to the appearance of the PPFS electronegative substituent, the signals of the ortho-protons of the CPDB and the PPFS-CTA are well separated, in contrast to the signals of the meta- and para-protons at 7.46 and 7.66 ppm CPDB, which are superimposed on the broadened signals of protons PPFS-CTA.
The dependences of q and qCPDB on the reaction time are shown in Fig. 3 (a, b). From Fig. 3b, it can be seen that at the beginning of the reaction (in the time interval from 0 to ~ 80 min), the consumption rates of the monomer and the CPDB practically coincide, which supports the earlier assumption that the signals at 7.98 and 7.93 ppm characterize the adduct of the CPDB and the PFS.
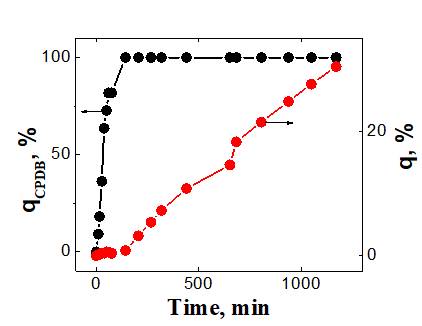
(a)
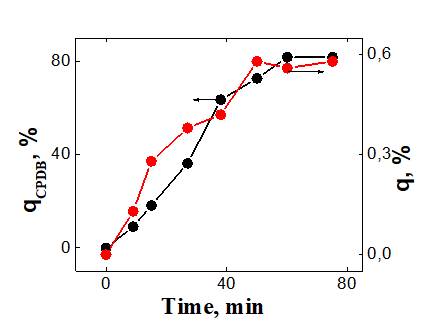
(b)
Fig. 3. The dependence of the conversion of CPDB and PFS on the polymerization time is up to 1170 minutes (a) and up to 142 minutes (b). [PPS] = 2 mol / l, [AIBN] = 2.7 × 10-3 mol / l, [CPDB] = 5.3 × 10-3 mol / l, 50 °С, DMF-d7.
To quantify the Ctr, q and qCPTB were used, calculated at the earliest conversions, when the contribution of the macro-CTA to chain formation can be neglected. Ctr was determined by the slope of the dependence of ln [CPDB] = f (ln [PFS]), obtained by logarithmizing the equation (2). The logarithmic dependence of the concentration of CPDB on the concentration of PFS is shown in Fig. 4. The estimation of the transfer constant at the CPDB, carried out for polymerization of PFS at conversion below 1%, gives the value Ctr = 77.4 2.2. Since the found value of Ctr >> 1, then the CPDB can be considered an effective CTA in the polymerization of PFS.
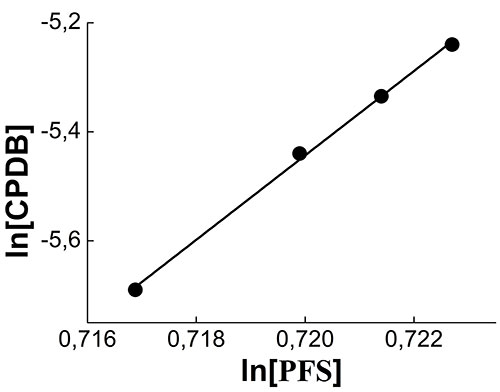
Fig. 4. The logarithmic dependence of [CPDB] on [PFS] during polymerization in DMF-d7 at 50 °С. [PFS] = 2 mol / l, [AIBN] = 2.7 × 10-3 mol / l, [CPDB] = 5.3 × 10-3 mol / l
Conclusion
The polymerization of PFS was studied in the presence of CPDB in situ using 1H-NMR spectroscopy. It has been established that the CPDB is consumed completely in the early stages of polymerization of PFS at q = 0.83% and is an effective CTA with Ctr = 77.4 2.2. Monitoring the polymerization of PFS in the presence of the CPDB using 1H-NMR spectroscopy allows determining the conversion of the CPDB and the monomer at a given time, making the value of the effective transfer constant determined in this work reliable. The presence of the induction period may be due to the formation of an adduct of the CPDB with PFS.
The project was financially supported by Russian Science Foundation, Grant No 17-13-01359.
The structure of the compounds obtained was studied using the equipment of the Center for the Study of the Structure of Molecules of the INEOS RAS.
References
- Ma J., Cheng C., Sun G., Wooley K.L. Macromolecules. 2008, 41 (23), 9080-9089.
- Rowe M., Teo G.H., Horne J., Al-Khayat O., Neto C., Thickett S.C. Aust. J. Chem. 2016, 69 (7), 725-735.
- Riedel M., Stadermann J., Komber H., Simon F., Voit B. Eur. Polym. J. 2011, 47 (4), 675-684.
- Chekurov K.Е., Barabanova A.I., Blagodatskih I.V., Lokshin B.V., Peregudov A.S., Abramchuk S.S., Khokhlov A.R. Dokl. AN. 2019, 484 (4), 431-435. (in Russian)
- Brummelhuis N., Weck M. J. Polym. Sci. Pol. Chem. 2014, 52 (11), 1555-1559.
- Chekurov K.Е., Barabanova A.I., Blagodatskih I.V., Peregudov A.S., Khokhlov A.R. Fluorine notes. 2019, 123 (2), 1-2.
- Moad G., Rizzardo E., Thang S.H. Polymer 2008, 49, 1079-1131.
- Chong B.Y.K., Krstina J., Le T.P.T., Moad G., Postma A., Rizzardo E., Thang S.H. Macromolecules 2003, 36 (7), 2256-2272.
- Gromov V.F., Bogachev Yu.S., Bune Ye.V., Zhuravleva I.L., Teleshov E.N. Vysokomol. Soed. A. 1993, 35 (1),7-12. (in Russian)
- Gromov V.F., Bune Ye.V., Barabanova A.I., Kozlova N.V., Zhuravleva I.L., Teleshov E.N. Vysokomol. Soed. A.. 1995, 37 (11), 1818-1822. (in Russian)
- Bune Ye.V., Barabanova A.I., Bogachev Yu.S., Gromov V.F. Eur. Polym. J. 1997, 33 (8), 1313-1323.
- Klumperman B., van den Dungen E. T. A., Heuts J. P. A., Michael J. Monteiro M. J. Macromol. Rapid Commun. 2010, 31, 1846–1862.
Recommended for publication by Prof. S. M. Igoumnov
Fluorine Notes, 2019, 124, 1-2
