Received: December 2018
DOI 10.17677/fn20714807.2019.03.02
Fluorine Notes, 2019, 124, 3-4
About the Mechanism of Cationic Polymerization of P-Ethylstyrene in the Presence of a Complex Catalyst Boron Fluoride – Water
V.A. Babkin1, D.S. Andreev1, A.V. Ignatov1, L.M. Lisina1, E.S. Titova2,3, A.R. Denisyuk4, A.I. Rakhimov2,V.T. Fomichev5, K.Yu. Prochukhan6, G.E. Zaikov7
1Sebryakovsky branch of the Volgograd State Technical University, 403343 Volgograd Region, Mikhaylovka, Michurin Street,21
e-mail: babkin_v.a@mail.ru
2Volgograd State Technical University, 400131 Volgograd, Lenin Avenue, 28
e-mail: organic@vstu.ru
3Volgograd State Medical University, 400131 Volgograd, Square Pavshich bortcov, 1
4Medical College of Volgograd State Medical University, 400001 Volgograd, Kim Street, 18
5Institute of Architecture and Building of Volgograd State Technical University, 400074 Volgograd, Akademicheskaya Street, 1.
6Bashkir State University, 450076 Republic of Bashkortostan, Ufa, Zaiki Validi Street, 32
7Institute of Biochemical Physics, 119334 Moscow, Kosugin Street, 4
Abstract: A quantum-chemical study of the initiation mechanism for the cationic polymerization of p-ethylstyrene in the presence of a complex catalyst boron fluoride - water by the classical ab initio method in the basis of 6-311G** was first performed. Geometry optimization was performed for all parameters by the standard gradient method. The studied reaction has a barrier character and is exothermic. The energy barrier in the attack of the initiating particle on the α-carbon atom of isoolefin is 262 kJ/mol, the thermal effect of the reaction is 39 kJ/mol.
Keywords: p-ethylstyrene, boron fluoride-water catalyst, initiation mechanism, ab initio quantum chemical method, energy barrier, thermal reaction effect.
Introduction
Boron fluoride aquacomplexes are classic catalysts for the cationic polymerization of olefins [1]. The mechanisms of elementary acts (initiation and growth) in the presence of these catalysts have so far been studied only for ethylene, propylene, and isobutylene [2]. For other olefins (linear, branched, styrene, etc.), these mechanisms have not yet been studied. The study of the initiation mechanism is very important, since at this stage it is possible to control the polymerization process. This is especially true of mechanisms at the electronic nanoscale. In this connection, the purpose of this work is to study the mechanism of isoolefin p-ethylstyrene initiation in the presence of boron fluoride aquacomplex.
Methodical Part
The mechanism of initiation of the cationic polymerization of p-ethylstyrene in the presence of a complex catalyst was studied according to the procedure described in [3-4]. The software was used the same as in [5-7] for calculation using the ab initio / 6-311G ** method [8]. As the reaction coordinates, the bond lengths of the studied molecular systems RC1H24 and RC2O23 were chosen (Fig. 1).
Results and Discussion
The mechanism of p-ethylstyrene initiation in the presence of the BF3·H2O complex catalyst at the attack of the α-carbon atom is shown in Fig. 1-5. In Fig. 1 shows the initial model of the interaction of p-ethylstyrene with the BF3·H2O complex catalyst at the attack of an initiating particle (proton H24) of the monomer. In Fig. 2 shows the geometric and electronic structure of the active center. In Fig. 3. shows the surface interaction of the monomer with the catalyst BF3·H2O. Reaction energy profile illustrated at fig4. Fig. 5 shows a graph of charges on the atoms of the molecular system when the catalyst interacts with the monomer along the RC1H24 reaction path (kJ/mol. Fig. 6 shows the initial model of the interaction of p-ethylstyrene with the complex catalyst BF3.
The process of interaction of p-ethylstyrene with the BF3·H2O complex catalyst during an attack by the proton of the α-carbon monomer atom can be divided into 3 stages (similar to [2]): the first is the coordination stage (1-3 steps), the second is the π-bond breaking stage monomer (steps 4-17) and the third - the formation of the active center (AC) (18-23 steps). At the coordination stage, the reaction coordinates of RC1H24 and RC2O23 vary from 3,1 to 2,9 nm and 3,3 to 3,1 nm respectively. At this stage, the mutual orientation of the aquacomplex of boron fluoride and the monomer occurs, and the angle of attack of the initiating particle of the H24+ α-carbon atom is calculated. At the π-bond breakdown stage, the reaction coordinates of RC1H24 and RC2O23 vary from 2,8 to 1,7 nm and 3,0 to 2,0 nm, respectively. At the third stage, the reaction coordinates of RC1H24 and RC2O23 vary from 1,6 to 1,1 nm and from 1,9 to 1,5 nm, respectively; an active center is formed, which is a polarized intermediate –[BF3 ∙ OH]- … [C(1) CH3 ∙ C9H10] +. At the coordination stage, the approach of the monomer to the catalyst is energetically favorable, which is characterized by the minimum energy of the entire molecular system (E0). Starting from the 4th to the 17th stage, the value of E0 increases, and starting from the 18th stage, it begins to decrease sharply. The energy barrier of the reaction is 262 kJ/mol (Fig. 4). The decrease in the total energy of the system is directly related to the onset of the interaction of the initiating particle H+ with the α-carbon atom of p-ethylstyrene and the breaking of the π-bond. At the third stage of interaction, the total energy of the entire molecular system reaches its minimum, which indicates the complete formation of the active center. The reaction is exothermic and its thermal effect is 39 kJ/mol.
When an initiating particle of the α-carbon atom of p-ethylstyrene attacks, the charge on the hydrogen atom H24 varies within the coordinates of the reaction from 0,307 to 0,131. The charge on the C1 atom (α-carbon atom) changes during the reaction under study, from -0,169 at the coordination stage to -0,206. The maximum value of -0,433 is reached at the π-link break stage. The charge on the C2 atom at the π-bond breakage stage is negative and varies from -0,153 to -0,043. In the formed carbon cation, it is +0,19. The charge on the oxygen atom O17 during the reaction varied from -0,442 to -0,538, and in the final carbon cation model it is equal to -0,517. The remaining charges in the reaction process did not change significantly. In this regard, we considered only the changes in the charges on the atoms, which directly participated in the initiation reaction of p-ethylstyrene in the presence of BF3·H2O.
It is obvious that the mechanism of initiation of cationic polymerization of the monomer under study in the presence of an aquacomplex of boron fluoride has the features of coordinated interactions; during the reaction, two bonds, RC1H24 and RC2O23, are simultaneously broken and new bonds are formed - RC1H24 and RC2O23.
Conclusion
Thus, we performed a quantum-chemical calculation of the mechanism for initiating the cationic polymerization of p-ethylstyrene in the presence of a complex catalyst boron fluoride - water using the classical ab initio HF/6-311G** method for the first time. Geometry optimization was performed for all parameters by the standard gradient method at each interaction step in the course of attacks of an initiating particle on α-carbon monomer atoms. It is established that the reaction is of a barrier nature and is exothermic. The energy barrier during the attack of the initiating particle on the α-carbon isoolefin atom is 262 kJ/mol, the thermal effect of the reaction is 39 kJ/mol.
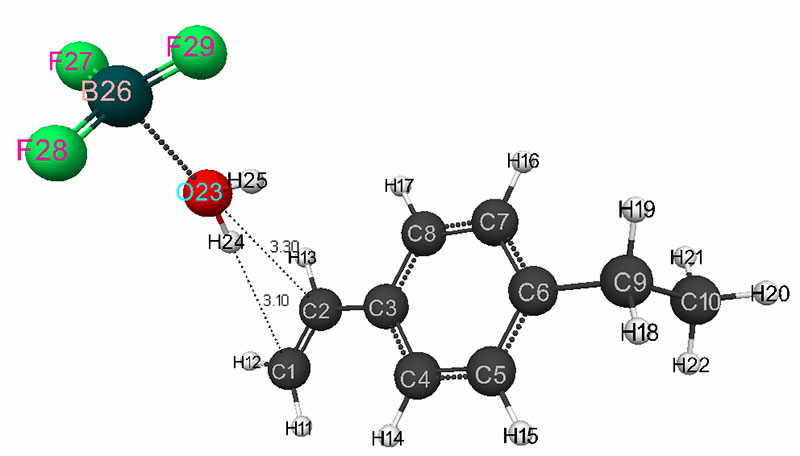
Fig. 1. The initial model of the interaction reaction of the H2O·BF3 complex catalyst with p-ethylstyrene.
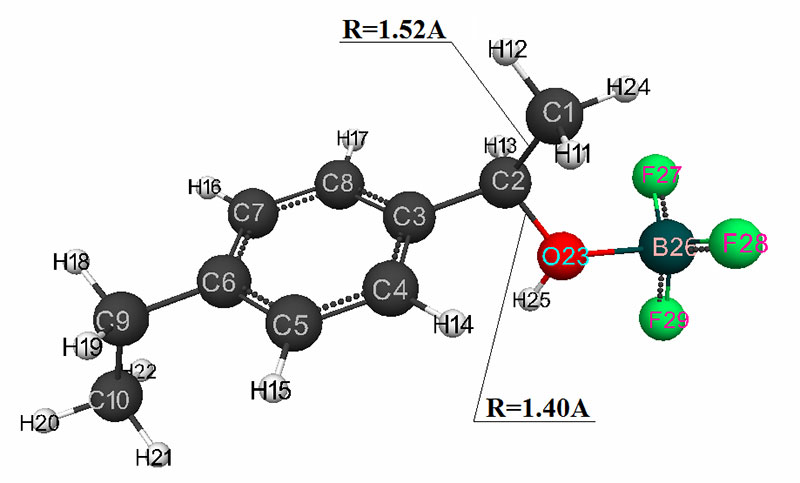
Fig. 2. The result of the interaction reaction of the H2O·BF3 complex catalyst with p-ethylstyrene.
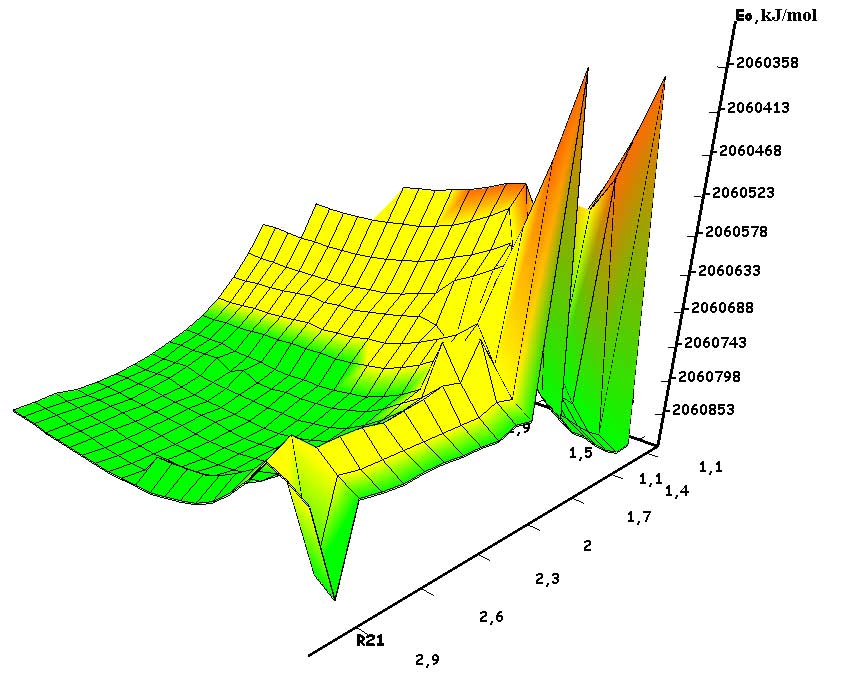
Fig. 3. Graph of the total energy change along the reaction path of the interaction of the H2O·BF3 complex catalyst with p-ethylstyrene.
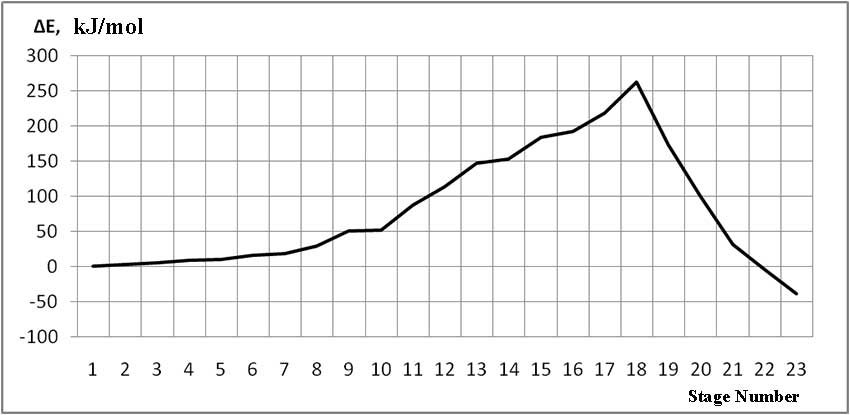
Fig. 4. Total energy change along the reaction path of the interaction of the H2O·BF3 complex catalyst with p-ethylstyrene.
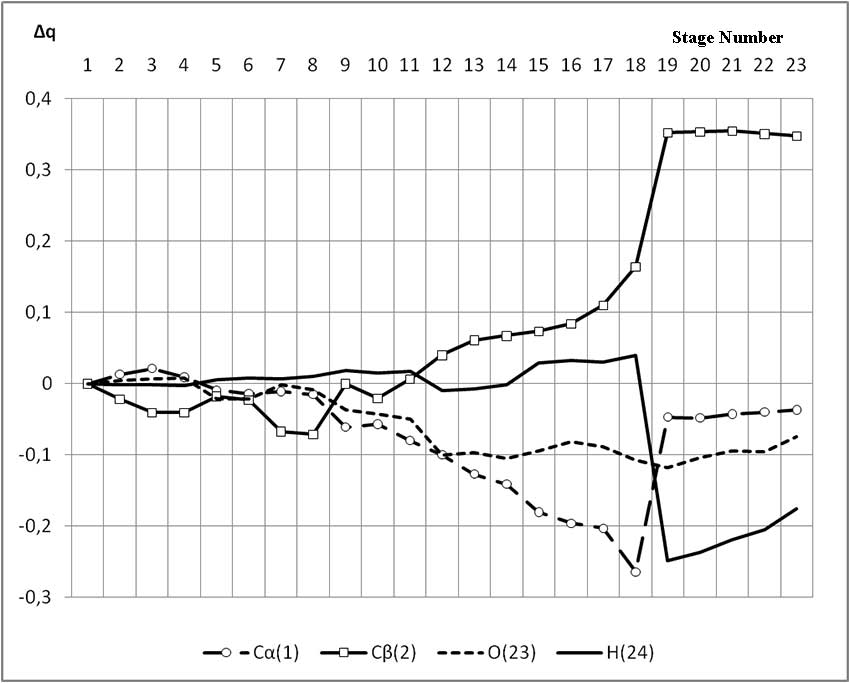
Fig. 5. Changes of some atoms charges along the reaction path of the interaction of the H2O·BF3 complex catalyst with p-ethylstyrene
References
- Kennedy J. Cationic polymerization of olefins, Moscow, 1978, 431 p.
- Babkin V.A., Zaikov G.E., Minsker K.S.. Quantum-chemical aspect of cationic polymerization of olefins. Ufa, Gilem, 1996. 188 p.
- Andreev D.S., Babkin V.A., Zaikov G.E. Quantum-chemical study of the mechanism of isoolefin initiation of o-methylstyrene in the presence of aluminum chloride aqua complex. Herald of Kazan Technological University, 2015, 18, 1, p. 28-31.
- Babkin V.A., Andreev D.S., Ignatov A.V., Rakhimov A.I, Titova E.S., Rakhimova O.S., Fomichev V.T. Quantum-chemical study of the initiation mechanism of the cationic polymerization of the 2-methylbutene-1 isoolefin in the presence of the boron fluoride aquacomplex. Fluorine Notes, 2018, 5(120), p. 3-4.
- Granovsky Alex A., Firefly version 8. http://classic.chem.msu.su/gran/firefly/index.html
- Bode B. M. and M. S. Gordon. MacMolPlt: A Graphical User Interface for GAMESS. Journal of Molecular Graphics. 1998, 16, 133-138.
- Schmidt M. W., Baldridge K. K., Boatz J. A., Elbert S. T., Gordon M. S, Jensen J. H., Koseki S., Matsunaga N., Nguyen K. A., Su S. J., Windus T. L., Dupuis M., Montgomery J. A. General Atomic and Molecular Electronic Structure System. Journal of Computational Chemistry, 1993, 14, 1347-1363. doi:10.1002/jcc.540141112.
- Tsirelson V.G. . Quantum Chemistry. Molecules, molecular systems and solids. Moscow, Binom, 2010, 496 p.
Recommended for publication by Prof. S. M. Igoumnov
Fluorine Notes, 2019, 124, 3-4
