Received: April 2019
DOI 10.17677/fn20714807.2019.03.05
Fluorine Notes, 2019, 124, 9-10
RAFT-COPOLYMERIZATION OF VINYL ACETATE AND 2,2,3,3,4,4,5,5-OCTAFLUOROPENTYLACRYLATE
A.O. Grigoreva, E.J. Polozov, S.D. Zaicev
Lobachevsky State University of Nizhny Novgorod, 603950, 23 Gagarina Avenue, Nizhnij Novgorod, Russia.
e-mail: alexx.grigoreva@gmail.com
Abstract: The possibility for obtainment of amphiphilic copolymers on the base of vinyl acetate and 2,2,3,3,4,4,5,5-octafluoropentylacrylate in the reversible chain transfer conditions, by addition-fragmentation mechanism, in the presence of low-molecular and high-molecular RAFT-agents, was studied. Molecular-mass characteristics and content of the obtained polymers were determined.
Keywords: fluoropolymers, RAFT-(co)polymerization, amphiphilic copolymers.
Introduction
Amphiphilic copolymers are a unique group of surface-active agents. Such polymers can be obtained using copolymerization of monomers which are not crystal-clear diphilic, but differ in hydrophilic properties. This approach offers exciting possibilities for macromolecular design. Polymerization with reversible chain deactivation methods development made possible an obtainment of copolymers with a given macromolecular architecture and narrow molecular-weight distribution. Among these methods, RAFT-(co)polymerization has a number of advantages, related to universality, efficiency and simpleness.
In the given research the copolymerization of vinyl acetate (VA) and 2,2,3,3,4,4,5,5-octafluoropentylacrylate (OFPA) in the presence of low-molecular and high-molecular RAFT-agents has been investigated.
Copolymers, on the base of vinyl acetate, draw attention due to their widespread application in various fields. Besides that, the usage of vinyl acetate elements hydrolysis allows to obtain polyvinyl alcohol (PVA). Hydrophilic nature of the vinyl alcohol elements allows to use vinyl acetate in amphiphilic copolymer synthesis. The high activity of the radicals, which are formed during vinyl acetate polymerization process, complicates its application in a polymerization with reversible chain deactivation because it often leads to a process inhibition or to a formation of polymers with a wide molecular weight distribution. Application of xanthates, as reversible chain transfer agents at PVA synthesis, turned out to be the best studied process [1-3]. The synthesis under high pressure [4] is one of the suggested approaches of vinyl acetate’s RAFT-polymerization. The possibility of trithiocarbonates and dithiobenzoates usage in polyvinyl alcohol controlled synthesis was studies in E.V. Chernikova’s and others article [5]. Regardless of the fact that synthesis of the different copolymers with vinyl acetate had been studied enough, the possibility of its copolymerization by the RAFT-mechanism with fluoroacrylates has not been investigated yet.
Fluorinated polymers, in turn, are of interest due to their properties, driven by fluorine strong electro negativity and its tight binding with carbon atom. They are characterized by thermal and chemical stability, weathering resistance, low surface energy and low refractive index [6]. Among fluoro(met)acrylates there was studied (co)polymerization by the method of reversible chain transfer for the next monomers: 2,2,3,3,4,4,4-heptafluoro-butylacrylate 1,1,1,3,3,3-hexafluoro-izopropylmetacrylate 2,2,3,4,4,4-hexafluorobutyl-metacrylate, 2,2,2-trifluoroethyl-metacrylate, 2-(perfluorohexyl)ethylmetacrylate, dodecafluoroheptylmetacrylate, 1,1,2,2-tetrahydroperfluorodecylacrylate, pentafluoro-phenylmetacrylate [7-9]. Octafluoropentylacrylate polymerization in the presence of benzyldithiobenzoate and its copolymerization with N-vinylpyrrolidone was investigated [10].
For our research, dibenzyltrithiocarbonate was chosen as an agent of reversible chain transfer due to the fact, that dibenzyltrithiocarbonates usually are used for polymerization of monomers of acrylic range, and also because earlier we have shown the efficiency of dibenzyltrithiocarbonate for 2,2,3,3,4,4,5,5-octafluoropentylacrylate homopolymerization,.
Experimental
Dibenzyltrithiocarbonate (was synthesized according to the described earlier methodology [11] and characterized by 1H NMR), 2,2′-azobisisobutyronitrile (AIBN, 98%, Sigma Aldrich), benzene, vinyl acetate (VA, 98%, Sigma Aldrich) and 2,2,3,3,4,4,5,5-octafluoropentylacrylate (OFPA, 99%, P&M Invest) were purified according to the overage methodologies. Chloroform-d (Cambridge Isotope Laboratories, D, 99.8%) was used without prepurification.
Calculated quantity of azobisisobutyronitrile and dibenzyltrithiocarbonate were dissolved in the mixture
of actafluoropentylacrylate, vinyl acetate and benzene (monomers and benzene volume ration was equal
6:1, [azobisisobutyronitrile] = 0.001 mmol/l,
f1(vinyl acetate) = 0.5). The mixture
was placed into Shlenk flask with rubber sept, was devolatilised by triple duplication of freeze-defrost
cycles, was placed into thermostat at 80°C with magnetic stirring. The mixture was being derived
with priming pulser at stated intervals, purified by hexane reentrainment from solution in THF, dried
under vacuum till the constant mass. Conversion was measured gravimetrically. Content of the obtained
copolymers was investigated by 1H NMR (Agilent DD2 NMR 400WB, 25°C).
For synthesis of the polymeric RAFT-agent on the base of octafluoropentylacrylate (poly-OFPA-СТА), 0.75 mmol of dibenzyltrithiocarbonate and 0.004 mmol of azobisisobutyronitrile were dissolved in a mixture of 4 ml of monomer and 0.5 ml of benzene. The mixture was placed into an ampoule, was devolatilised by triple duplication of freeze-defrost cycles, the ampoule was sealed off, placed in thermostat at 80°C. Polymerization was being held during 3 hours. The mixture was derived; polymer was purified by hexane reentrainment from solution in THF, dried under vacuum till the constant mass. Conversion was estimated gravimetrically (found76%). Mn = 4500, Ð = 1.37.
The study of molecular-mass characteristics was held using gel-permeation chromatography in THF, at 40 °C, on Prominence LC-20VP (Shimadzu) chromatograph supplied with differential refractometer and UV-detector. Polystyrene and PMMA (Fluka) tight-dispersive standards were used for calibration. Obtained chromatographs were processed in software “LCsolution”.
Results and discussion
Copolymerization of octafluoropentylacrylate and vinyl acetate was being held in the presence of dibenzyltrithiocarbonate
with a concentration 0.05 mole·l-1. The process flows with a low speed (significantly
lower than octafluoropentylacrylate homopolymerization process). Polymer’s conversion reaches 30%
in 2.5 hours and after it increases negligibly (after 11 hours of polymerization do not exceed 40
%). Such inhibition may be explained by high intermediate’s stability, formed by macroradical with
VA-end group (Scheme 1).
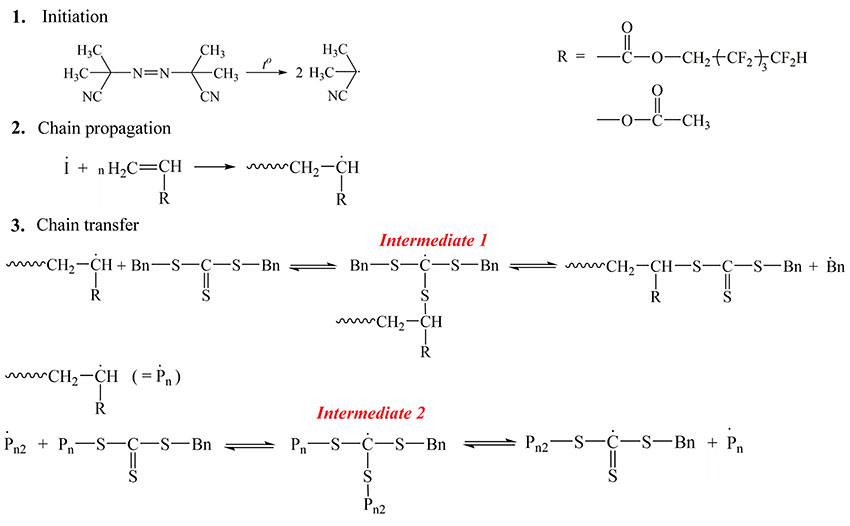
Scheme 1. Scheme for copolymerization of octafluoropentylacrylate and vinyl acetate in the presence of dibenzyltrithiocarbonate as an agent of reversible chain transfer and AIBN as an initiator.
The presence of vinyl acetate bonds in polymer was proved by 1H NMR (Pic. 1). The amount of
vinyl acetate in copolymer at a maximum conversion (40 %) is equal F1 (vinyl acetate)
= 0.17.
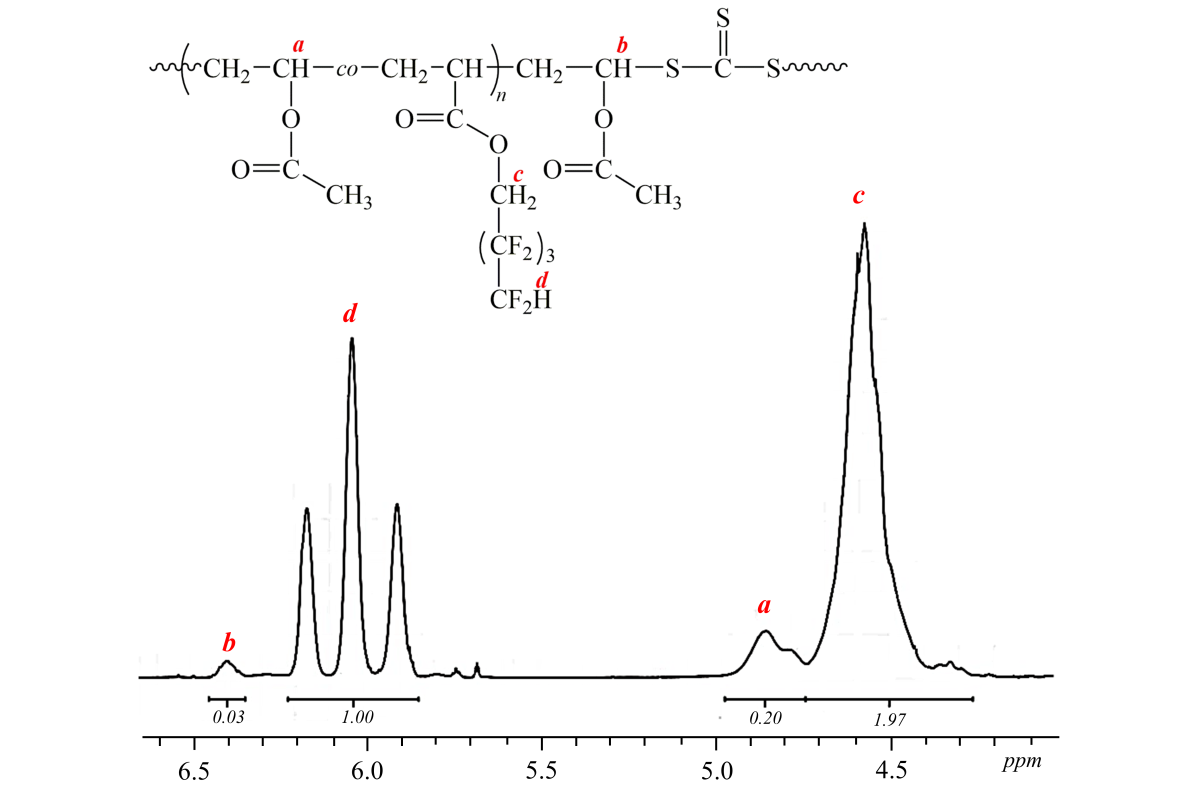
Picture 1. NMR of P(OFPA-co-VA) copolymer, obtained in the presence of dibenzyltrithiocarbonate at conversion 40 %
([BTC] = 0.05 mole·l-1, [AIBN] = 0.001 mole·l-1, f1(VA) = 0.5).
Gel-permeation chromatography curves of obtained copolymers are unimodal and shift to the high molecular weight field with the conversion raise, polydispersity decreases, what means that the process flows under control (Pic. 2). The presence of a «shoulder» on GPC-curve of copolymer with 8.1 % conversion corresponds to oligomeric RAFT-agent, which is probably formed due to cooperation of dibenzyltrithiocarbonate with vinyl acetate radical. Due to the fact that vinyl acetate is a worth leaving group then Bn and octafluoropentylacrylate, such oligomeric agent is spent less, than dibenzyltrithiocarbonate (at octafluoropentylacrylate homopolymerization, dibenzyltrithiocarbonate is nearly completely used at 8% conversion). As can be seen from the above, vinyl acetate participates in copolymerization from the earliest conversions.
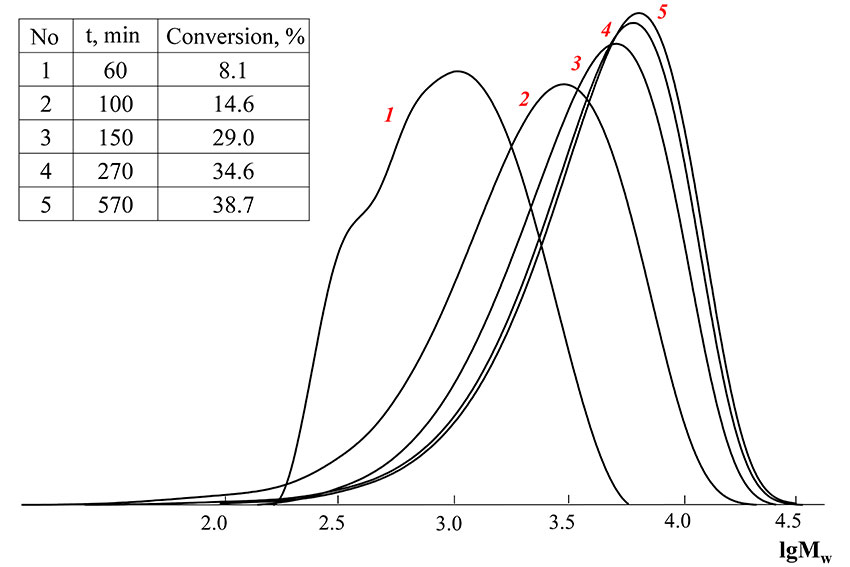
Picture 2. GPC-curves of the polymers, obtained by copolymerization of vinyl acetate and octafluoropentylacrylate in the presence of dibenzyltrithiocarbonate
([BTC] = 0.05 mole·l-1, [AIBN] = 0.001 mole·l-1, f1(VA) = 0.5)
In case of concentration of dibenzyltrithiocarbonate decrease up to 0.02 mole·l-1, instead of copolymerization the process of octafluoropentylacrylate homopolymerization takes place. This fact may be explained by the decrease of the possibility of cooperation between VA-macroradical with dibenzyltrithiocarbonate with the formation of a more stable intermediate. Consequently, there is no slowdown and the more active octafluoropentylacrylate is polymerized. While attempting to copolymerization in the presence of high-molecular RAFT-agent of POFPA, there is also a process of OFPA homopolymerization, what may be explained by preferential salvation of octafluoropentylacrylate-macroradical to «its» monomer.
Conclusion
Notwithstanding that controlled synthesis of vinyl acetate (co)polymers according to RAFT-mechanism is heavy, but it seems possible to obtain its copolymers with fluorinated monomer - octafluoropentylacrylate in the presence of dibenzyltrithiocarbonate under specified conditions. Such copolymers, at further hydrolysis of vinyl acetate monomer units, will show amphiphilic properties.
Acknowledgements
This work was supported by RFBR grant No 19-03-00843.
References
- R. Fleet, J. B. McLeary, V. Grumel, W. G. Weber, H. Matahwa, R. D. Sanderson. Preparation of New Multiarmed RAFT Agents for the Mediation of Vinyl Acetate Polymerization. Macromol. Symp. 2007, 255, 8–19.
- V. Malepu, C. D. Petruczok, T. Tran, T. Zhang, M. Thopasridharan, D. A. Shipp. RAFT Polymerization of Vinyl Acetate, Styrene and Acrylates Using N,N-Dithiocarbamates. ACS Symposium Series; American Chemical Society: Washington, DC, 2009.
- RAFT/MADIX miniemulsion polymerization of vinyl acetate: influence of oil soluble initiators, temperature, and type of chain transfer agent in nanodroplets. T. Segura, R. Peralta, M. Menes-Arzate, F. Leуn, R. Mendoza. Colloid and Polymer Science. 2017.
- J. Chen, X. Zhao, L. Zhang, Zh. Cheng, X. Zhu. Reversible Addition-Fragmentation Chain Transfer Polymerization of Vinyl Acetate Under High Pressure. Journal of Polymer Science, Part A: Polymer Chemistry. 2015, 53, 1430–1436.
- E. V. Chernikova, V. V. Yulusov, K. O. Mineeva, E. S. Garin. Pseudoliving polymerization of vinyl acetate mediated by reversible addition-fragmentation chain-transfer agents. Journal of Polymer Science, Part B. 2011, 53, 1433–1443.
- A. Bruno. Controlled Radical (Co)polymerization of Fluoromonomers. Macromolecules. 2010, 43, 10163–10184.
- B. P. Koiry, M. Moukwa, N. K. Singh. Reversible addition–fragmentation chain transfer (RAFT) polymerization of 2,2,3,3,4,4,4-heptafluorobutyl acrylate (HFBA). Journal of Fluorine Chemistry. 2013, 153, 137–142.
- B. P. Koiry, H. Klok, N. K. Singh. Copolymerization of 2,2,3,3,4,4,4-heptafluorobutyl acrylate with butyl acrylate via RAFT polymerization. Journal of Fluorine Chemistry. 2014, 16, 109–115.
- L. Nuhn, L, Overhoff, M. Sperner, K. Kaltenberg, R. Zentel. RAFT-polymerized poly(hexafluoroisopropylmethacrylate)s as precursors for functional water-soluble polymers, Polym. Chem. 2014, 5, 2484-2495.
- S. D. Zaitsev, Yu. D. Semchikov, E. V. Vasil’eva, L. V. Kurushina. Controlled radical (co)polymerization of (meth)acrylic esters under reversible chain transfer conditions, Polymer Science Series B. 2012, 54, 205-214.
- E. V. Chernikova, P. S. Terpugova, E. S. Garina, and V. B. Golubev. Controlled Radical Polymerization of Styrene and n-Butyl Acrylate Mediated by Trithiocarbonates, Polymer Science Series A. 2007, 49, 108-119.
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2019, 124, 9-10
