Received: June 2019
DOI 10.17677/fn20714807.2019.04.02
Fluorine Notes, 2019, 125, 3-4
SYNTHESIS AND ANTITUMOR ACTIVITY OF 1-(1,1,1,3,3,3-HEXAFLUORO-2-FERROCENYLPROPAN-2-YL)-1H-IMIDAZOLE
A.A. Simenel1,2,V. I. Dyachenko1*, and S.M.Igumnov1,3,
1A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,
Vavilova str. 28, V-334, GSP-1, 119991 Moscow, Russia
2National University of Scienceand Technology "MISIS", Chemistry Department, 4 Leninskiy
Ave., 119049 Moscow, Russia
3NPO PIM-INVEST LLC, Vavilova, 28, Moscow,119991, Russia
е-mail:vic-d.60@mail.ru
Abstract: 1-(1,1,1,3,3,3-Hexafluoro-2-ferrocenylpropan-2-yl)-1H-imidazole (3)was synthesized according to 2 methods: interaction of 1,1,1,3,3,3-hexafluoro-2-ferrocenylpropan-2-ol with N,N-carbonyldiimidazole (CDI) in boiling toluene, and substitution of chlorine by imidazole in 1-(2-chloro-1,1,1,3,3,3-hexafluoropropan-2-yl)ferrocene in presence of KI. In experiments in vivo antitumor effects of (3) against murine solid tumor system Ca755 carcinoma were evaluated.
Keywords: ferrocene, imidazole, 1,1,1,3,3,3-hexafluoro-2-ferrocenylpropan-2-ol, 1-(2-chloro-1,1,1,3,3,3-hexafluoropropan-2-yl)ferrocene, antitumor activity.
Incorporation of ferrocene moiety into organic compounds leads to different biological activity which is associated with abnormal biodegradation of ferrocene compounds. Ferrocene-containing heterocyclic compounds with great success have proved themselves as potential medicines for the treatment of diseases of various origins [1-3]. Moreover ferrocene derivatives of heterocycles were shown to display pronounced antitumor activity in combination with low toxicity, which was shown either in vitro or in vivo, in models of transplantable solid tumors [4-6]. At the same time the introduction of fluorine or perflourinated alkyl substituents into biologically active compounds is an important method of synthesis of potential drugs. Thus, the introduction of fluorine into biologically active compounds leads to an increase in their lipophilicity and improved penetration through the cell membrane [7]. Their inclusion in the metabolism also contributes to the "masking effect" associated with the proportionality of the atoms of hydrogen and fluorine, and the comparability of the electronegativity of fluorine and oxygen [8].
The purpose of this work is to search for new methods for the synthesis of fluorine-containing ferrocenylazoles based on commercially available ferrocene and hexafluoroacetone and to study their antitumor activity. 1,1,1,3,3,3-Hexafluoro-2-ferrocenyl-propan-2-ol (1) (see Scheme 1), which is easily obtained as a result of their interaction, could become the starting point of the synthetic plan of target compound (3).
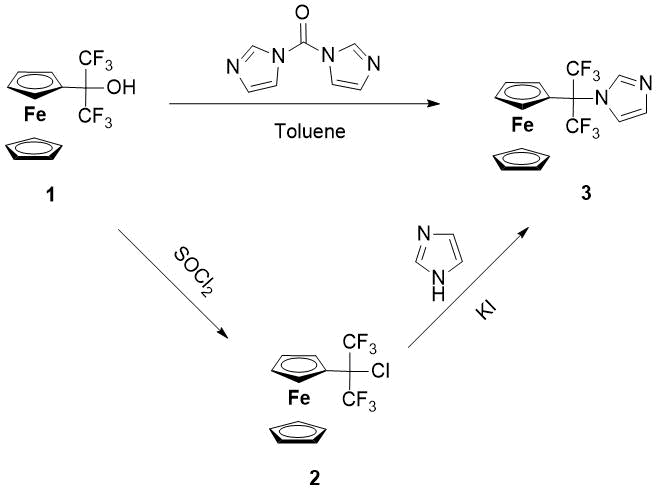
Scheme 1.Synthesis of 1-(1,1,1,3,3,3-hexafluoro-2-ferrocenylpropan-2-yl)-1H-imidazole (3)
A convenient synthetic method for the production of heterocyclic ferrocene derivatives is the alkylation of azoles by hydroxy(alkyl)ferrocenes in a two-phase aqueous-organic system (water-methylene chloride) during the catalysis with a strong mineral non-oxidizing acid (for example HBF4). Usually, the protonation of a hydroxyl group under these conditions leads to the formation of thermodynamically stable ferrocenyl carbocations under mild conditions, capable of N-alkylation of various heterocyclic compounds. It turned out that in the case of carbinol 1, the cation is not formed due to the destabilizing electron-withdrawing effect of the two CF3-groups. In this regard, we have adapted to Scheme 1 other methods of synthesizing compound 3.
1-(1,1,1,3,3,3-Hexafluoro-2-ferrocenylpropan-2-yl) -1H-imidazole (3) is formed in 71% yield with the interaction 1,1,1,3,3,3-hexafluoro-2-ferrocenyl propan-2-ol (1) and a 20% excess of 1,1-carbonyldiimidazole in boiling anhydrous toluene for three hours.
The first stable 1-(1,1,1,3,3,3,3-hexafluoro-2-chloropropan-2-yl)-ferrocene (2) [9] that we obtained by acting on carbinol 1 with thionyl chloride formed the basis of the second synthetic method of compound 3. Its essence lies in the nucleophilic substitution of chlorine with imidazole in the presence of potassium tert-butylate and a slight excess of KI. So, when boiling the source reagents in anhydrous toluene for 24 hours, the target 1-(1,1,1,3,3,3-hexafluoro-2-chloroprop-2-yl)-ferrocene is formed with a yield of 73%.
An in vivo study of the acute toxicity and anti activity of 1-(1,1,1,3,3,3-hexafluoro-2-ferrocenylpropan-2-yl) imidazole (3) was conducted in mice (model of solid tumor carcinoma Ca-755). Compound (3) was shown to have an antitumor effect against carcinoma-755, causing its growth to be inhibited by 80% (2) compared to control (1) (see Fig. 1).
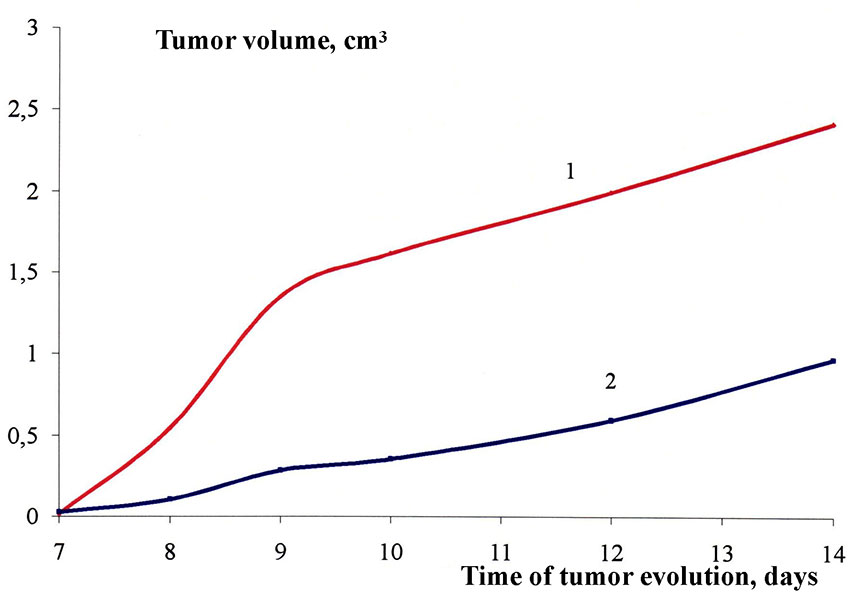
Fig.1. The effect of 1,1,1,3,3,3-hexafluoro-2-ferrocenylpropan-2-yl) imidazole 3 on suppressing the development of carcinoma 755 (2); control (1)
The appearance of animals treated with the drug, their appetite, activity, mobility, behavioral reactions, activity of the gastrointestinal tract corresponded to the norm and testified to the physically vigorous, healthy state of the mice. It should be particularly noted that compound 3 when administered orally in the dose range of 400–800 mg/kg is not only well tolerated by animals, but also causes a significant (67–98%) increase in their body mass compared with the control.
The observed sensitivity of Ca-755 carcinoma to the compound (3) with good tolerability of this compound by animals (MD = 800 mg/kg), as well as a significant increase in their body mass, indicates the need for further in-depth study of the mechanism of antitumor action and other pharmacological properties of fluoroalkyl-containing ferrocenes of this type
Experimental
EI mass spectra were taken on a FINNIGAN POLARIS Q spectrometer at 70 eV and the temperature of the ion chamber 250°C. 1H, 13C and 19F NMR spectra were obtained on a Bruker Avance 400 spectrometer (at 400, 100 and 375 MHz, respectively) in DMSO-d6 and CDCl3 at 30°C. The chemical shifts are given in the δ scale relative to the residual solvent signal, 19F according to CF3CO2H (external standard). Spin-spin interaction constants are given in MHz. Melting points were determined with a Stuart SMP30 apparatus (Bibby Scientific). Commercially available imidazole and N,N-carbonyldiimidazole (CDI) (Acros Organics) were used as purchased. 1,1,1,3,3,3-Hexafluoro-2-ferrocenylpropan-2-ol (1) and 1-(1,1,1,3,3,3-hexafluoro-2-chloropropan-2-yl)ferrocene were synthesized as described earlier methods [9].
Synthesis of 1-(1,1,1,3,3,3-hexafluoro-2-ferrocenylpropan-2-yl)-1H-imidazole (3)
Method 1. A mixture of 0.352 g of 1 mmol of 1,1,1,3,3,3-hexafluoro-2-ferrocenylpropan-2-ol (1) and 0.18 g (1.2 mmol) of N,N-carbonyldiimidazole was boiled in anhydrous toluene for three hours. After cooling the reaction mass, 50 ml of ether was added and resulting mixture was extracted with 20% phosphoric acid solution (2 × 50 ml). The combined aqueous fractions were alkalinized to pH 5 and extracted with methylene chloride (2 × 50 ml). The organic portion is dried over anhydrous sodium sulphate. The solvent was removed. The product was purified by column chromatography on silica gel, eluent - methylene chloride, 0.285 g of compound 3 was obtained. Yield 71%.
Method 2. A mixture of 0.370 g (1 mmol) of 1- [1,1,1,3,3,3-hexafluoro-2-chloropropan-2-yl]-ferrocene (2), 0.102 g (1.2 mmol) of imidazole, 0.135 g (1.2 mmol) of potassium t-butoxide and anhydrous potassium iodide (0.12 mmol) was boiled in anhydrous toluene for 24 hours. The reaction mass was cooled, the solvent was removed; the residue was purified by column chromatography on silica gel, eluent - methylene chloride. After removal of the solvent in vacuo, 0.294 g of compound 3 was obtained as a red-brown oil, which crystallizes on cooling, mp. 30-31°С. Yield 73%.
1-(1,1,1,3,3,3-Hexafluoro-2-ferrocenylpropan-2-yl)-1H-imidazole (3). M.p. 30-31°С, mass-spectra, m/z: (%): 402 (100) [M]+, 335 (55) [M-Im]+. 1Н NMR spectra, (δ, ppm): 4,03-4,29 (m, 9H, Fc), 4,61 (q, 1H, CH) 7,11 (s, 1Н, СН); 7,19 (s, 1Н, СН); 7,47 (s, 1Н, СН).19F NMR spectra, (δ, ppm): –7.31(s). 13C NMR spectra, (δ, ppm): 68.36 (Cp); 69.24 (Cp); 70.01 (Cp);70.28 (q, 2J=31); 79.68 (ipso-Cp);118.65 (Im(C-4));119.05 (Im(C-5));121.71 (q, CF3, 1J= 287); 136.25 (Im(C-2)).Calc. for C16H12F6FeN2:C, 47.79; H, 3.01; F, 28.35; Fe, 13.89; N, 6.97. Found: C, 47.40; H, 3.07; Fe, 13.76; N, 6.70;
The study of antitumor activity of1- (1,1,1,3,3,3-hexafluoro-2-ferrocenylpropyl-2) -1H-imidazole (3)
Carcinoma 755 (Ca755) was transplanted subcutaneously to theinbred mice f1(C57Bl/6xDBA2) - BDF1 males (60 animals), with weight 20-22 g, in accordance with the standard procedure. The compound (3) was administered on the next day after tumor inoculation. The tested daily doses were 10.0 and 20.0 mg kg-1. Oil solution (10 mg ml-1) of compound (3) were administered intraperitoneally starting from the next day after tumor inoculation. Each group comprised five to seven animals, including the control group of animals. The kinetics of tumor growth was studied by measurement of tumor size during the whole period of tumor development. Two cross-coupling tumor sizes were measured and the volume of thetumor was calculated as V=ab2/2, where a is the length and b is the width and the height of the tumor. As estimated previously, the density of tumor tissue is equal to 1.0 g cm3. So it is assumed that the weight of tumor in grams is equal to the volume of tumor in cm3. The index of tumor growth inhibition (TGI) was calculated as(C-T)/C, %, where C and T are the mean tumor weight in groups of control and treated animals, respectively.
Thus, in in vivo experiments, it was shown that compound 3 has a pronounced antitumor effect on carcinoma-755, causing growth inhibition by 80% compared with the control.
Study of acute toxicity of 1- (1,1,1,3,3,3-hexafluoro-2-ferrocenylpropyl-2)-1H-imidazole (3).
The acute toxicity of compound 3 was determined by single oral administration to BDF1 intact mice in the dose range from 400 to 800 mg / kg. The compound was administered to animals as a solution in sunflower oil at a concentration of 10 mg/ml. The volume of the orally administered solution of compound 3 varied, depending on the dose, from 0.8 ml to 1.6 ml.
In the studied dose range, compound 3 did not cause the death of animals while observing them for one month, which makes it possible to attribute it to the category of low-toxic substances.
Conclusion
- Interaction of 1,1,1,3,3,3-hexafluoro-2-ferrocenylpropan-2-ol with N,N-carbonyldiimidazole (CDI) in boiling toluene, as well as nucleophilic substitution of chlorine with imidazole in 1- (2-chloro-1,1,1,3,3,3-hexafluoropropan-2-yl)-ferrocene in the presence of KI leads to the formation of 1-(1,1,1,3,3,3-hexafluoro-2-ferrocenylpropan-2-yl)-1H-imidazole (3) with high yields.
- In in vivo experiments on mice, compound 3 was shown to have an antitumor effect on Ca-755 carcinoma, causing its growth to be inhibited by 80% compared to control.
- The low toxicity of CF3-containing ferrocenylimidazole 3, its good tolerability by animals, as well as a significant (1.6–2 times) increase in their body mass, which is not typical of therapy with anticancer drugs, indicates the need for further in-depth study of this type of compounds.
Acnowledgement
This work was supported by Ministry of Science and Higher Education of the Russian Federation using the equipment of Center for molecular composition studies of A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow.
Literature
- E. W. Neuse, J. Inorg. Organomet. Polymer. Mater. 2005, 15 (1), 3.
- V. N. Babin, Yu A. Belousov, V. I. Borisov, V. V. Gumenyuk, Yu. S. Nekrasov, L. A. Ostrovskaya, I. K. Sviridova, N. S. Sergeeva; A. A. Simenel and L. V. Snegur. Russ. Chem. Bull. 2014, 63, 2405.
- F. A. Larik, A. Saeed, T. A. Fattah, U. Muqadar, P. A. Channar. Appl Organometal Chem. 2017, 31, 1.
- L. V. Snegur, S.I. Zykova, A. A. Simenel, Y. S. Nekrasov, Z. A. Starikova, S. M. Peregudova, M.M. Ilyin, V.V. Kachala, I.K. Sviridova, N.S. Sergeeva. Russ. Chem. Bull. 2013, 62, 2056.
- L. V. Snegur, Yu. S. Nekrasov, N. S. Sergeeva, Zh V. Zhilina, V. V. Gumenyuk, Z. A. Starikova, A. A. Simenel, N. B. Morozova, I. K. Sviridova and V. N. Babin. Appl. Organomet. Chem. 2008, 22, 139.
- G. Jaouen, A. Vessières, S. Top. Chem. Soc. Rev. 2015, 44, 8802.
- Hans-Joachim Böhm, David Banner, Stefanie Bendels, Manfred Kansy,Bernd Kuhn, Klaus Müller, Ulrike Obst-Sander, and Martin Stahl, Fluorine in Medicinal Chemistry, ChemBioChem 2004, 5, 637-643. DOI: 10.1002/cbic.200301023
- R. Filler, in «Organofluorine Chemicals and Their Industrial Applications», ed. by R. E. Banks, Ellis Horwood, Ltd.,Chichester, UK (1979), Chap. 6.
- V. I. Dyachenko, A. S. Peregudov, I. V. Anan´ev, M. Yu. Antipin, Yu. S. Nekrasov,V. I. Sokolov, and S. M. Igumnov. Russ. Chem. Bull., 2011, 60, 764.
ARTICLE INFO
Received 13 June 2019
Accepted 15 July 2019
Available online August 2019
Recommended for publication by Prof. Vadim Kornilov
Fluorine Notes, 2019, 125, 3-4
