Received: April 2019
DOI 10.17677/fn20714807.2019.04.04
Fluorine Notes, 2019, 125, 7-8
LINEAR-DENDRITIC BLOCK COPOLYMERS BASED ON N-ISOPROPYL ACRYLAMIDE AND TRIS-(PENTAFTORPHENYL)HERMANE
O.S. Ilyanova, O.G. Zamyshlyayeva
National Research Nizhny Novgorod State University. N.I. Lobachevsky, 603950 Nizhny Novgorod, 23 Gagarin Ave.
e-mail: zam.olga@mail.ru
Abstract: The possibility of synthesizing amphiphilic linear-dendritic block copolymers due to the chain transfer reaction to tris-(pentafluorophenyl)germane during polymerization of N-isopropylacrylamide and subsequent activated polycondensation of the selected product with tris-(pentafluorophenyl)germanе is shown.
Keywords: chain transfer reaction, activated polycondensation, block copolymers, functional polymers, linear dendritic polymers.
Introduction
Recently, special attention has been attracted by "smart" polymers that are sensitive to changes in the external environment (temperature changes, pH or the introduction of additives). For them, a first-order phase transition occurs in solutions, accompanied by a sharp decrease in the specific volume of the macromolecule.
The largest number of studies for this group of polymers is devoted to poly-N-isopropylacrylamide (PNIAA). Its ability to reversibly transfer from a loose globule to a compact ball in solutions is used to make materials for biotechnological purposes, matrices for controlled drug delivery, dehydration of suspensions, concentration of protein solutions, etc. [1-2].
The most convenient method of chemical modification of PNIAA is its copolymerization, in which the change in the ratio of hydrophilic and hydrophobic groups is possible, which can lead to a change in the value of the lowest critical solution temperature (LCST), and to the appearance of new properties at interphase boundaries. The block copolymerization with fluorinated compounds, for example with polyphenylene germanе (PPG), is a rather effective method in this ratio, in which polymers with a linear-dendritic structure are obtained [3-6].
Earlier, a technique was developed for the synthesis of linear-dendritic block copolymers, including radical polymerization of vinyl monomers (methyl methacrylate (MMA), styrene (St), methyl acrylate (MA), 2,2,3,3-tetrafluoropropyl methacrylate (FMA), N- vinylpyrrolidone (N-VP)) in the presence of (C6F5)2GeH2 and (C6F5)3GeH and the subsequent activated polycondensation with tris-(pentafluorophenyl)germane. It was also shown that the preparation of linear dendritic block copolymers is possible when using as a starting compound a functional polymer PNIAA containing a bis(pentafluorophenyl)germane group at the end of the chain [7].
The effectiveness of the radical polymerization reaction in the preparation of functional polymers of various nature is ensured by high values of the relative chain transfer constants for organo-germanium compounds (Table 1) [3-6].
Table 1. The values of the relative chain transfer constants during the polymerization of some monomers.
|
Monomer |
СS |
|
|
(С6F5)2GeH2 |
(С6F5)3GeH |
|
|
St |
3.0 |
3.4 |
|
ММА |
0.87 |
0.3 |
|
N-VP |
1.8 |
0.02 |
|
FMA |
0.26 |
3.87 |
The nature of the vinyl monomer affects the structure of linear-dendritic block copolymers [3-6]. Due to the chain transfer reaction on (C6F5)2GeH2 and (C6F5)3GeH, the corresponding functional group appears at the end of the growing macromolecule. Further, 2 mechanisms of chain breakage are possible - disproportionation and recombination. It is the ratio of these reactions during radical polymerization that leads to the formation of tadpole and dumbbell structures, that is, the growth of a hyperbranched block on a functional polymer will occur in one or two directions, respectively [8].
The purpose of this work is to establish the possibility of the reaction of chain transfer to tris-(pentafluorophenyl)germane during polymerization of N-isopropylacrylamide and to obtain an amphiphilic linear-dendritic block copolymer based on N-isopropylacrylamide and perfluorinated polyphenylenegermane.
Experimental
The monomer used was N-isopropylacrylamide (Aldrich, 97%) was purified by recrystallization twice from hexane and dried in vacuum at room temperature. The initiator AIBN was purified by double recrystallization from isopropyl alcohol. The chain transmitter is tris-(pentafluorophenyl)germane, obtained at the Institute of Organometallic Chemistry G.A. Razuvayev, Russian Academy of Sciences, was recrystallized from hexane. Solvents used: hexane, acetone, benzene, tetrahydrofuran were purified by distillation at atmospheric pressure [9].
Radical polymerization NIAA
NIAA radical polymerization is conducted in the presence of the initiator AIBN (7.9*10-3 mol/l) in a solvent mixture of benzene:acetone=1:1, [10]. The concentration of the transmitter chain (C6F5)3GeH was changed in the range of 0-0.02 mol/l. The polymerization was carried out in dilatomer ampoules, which were frozen three times before freezing in vacuo. The reaction was carried out for 24 hours at 60°C. To isolate the polymers, a solvent-precipitator: acetone-hexane system was used. The relative constant of chain transfer during the polymerization of NIAA on (C6F5)3GeH was determined by the Mayo method: by the slope of the straight line in coordinates 1/p – [S]/[M] [11].
Activated polycondensation
The functional polymer PNIAA‒Ge(C6F5)3, obtained by radical polymerization of NIAA and 2·10-2 mol/l (С6F5)3GeH, was used for activated polycondensation with (С6F5)3GeH in the presence of Et3N in an МЭК solution in an Ar atmosphere [12].
For carrying out the activated polycondensation reaction, 2 solutions were prepared. Solution 1: 5% solution PNIAA‒Ge(C6F5)3 in MEK. Solution 2: 10% solution (С6F5)3GeH in MEK (m((С6F5)3GeH) = 2m(PNIAA‒Ge(C6F5)3). Solution 2 was poured into solution 1. Then Et3N (three-fold excess by moles relative to (C6F5)3GeH) was diluted in MEK (1:10 ratio) and added dropwise to the resulting mixture for 12 minutes in an argon atmosphere with constant stirring. The synthesis time is 1 hour. The resulting product was purified by reprecipitation using a solvent: precipitating system - THF: hexane. The selected polymer was dried in vacuum to constant weight. Since under these conditions PPG can be formed due to the reaction of activated polycondensation (С6F5)3GeH, the reaction product was purified by hot extraction in a Soxhlet extractor in THF for 4.5 hours to isolate the block copolymer. The polymer obtained after extraction was dried in vacuum to constant weight. The yield of linear-dendritic block copolymer was 21%.
The study obtained functional and linear dendritic block copolymers
The characteristic viscosity of polymer solutions was determined in THF at 27°C on a Ubbelohde viscometer (the elapsed time of the solvent t0=77.8 s). The average viscosity ММ (М) of the samples was determined using the Mark-Kun-Houwink equation: [η]= K(Mη)α (K = 9.59·10-3, α = 0.65 [10]).
The formation of the functional polymer PNIAA‒Ge(С6F5)3, as well as the hybrid linear dendritic block copolymer PNIAA‒PPG, was confirmed by IR and 19F NMR spectroscopy.
IR spectra were taken in KBr tablets, on an InfralumFT-801 IR spectrometer. NMR spectra were recorded on a Bruker Avance III 400 spectrometer, T=25°C. Deuterated chloroform was used as a solvent. Spectra were processed using the MestReNova software.
Results and discussion
During radical polymerization and subsequent activated polycondensation, NIAA homopolymer, a functional polymer containing tris-(pentafluorophenyl) germane groups at the end of the chain, and the block copolymer PNIAA–PPG were obtained.
The obtained value of the relative chain transfer constant during radical polymerization of NIAA to tris-(pentafluorophenyl) germane was 2.08, which allows us to conclude that this germanium-organic compound is a good chain transmitter (Cs ˃1).
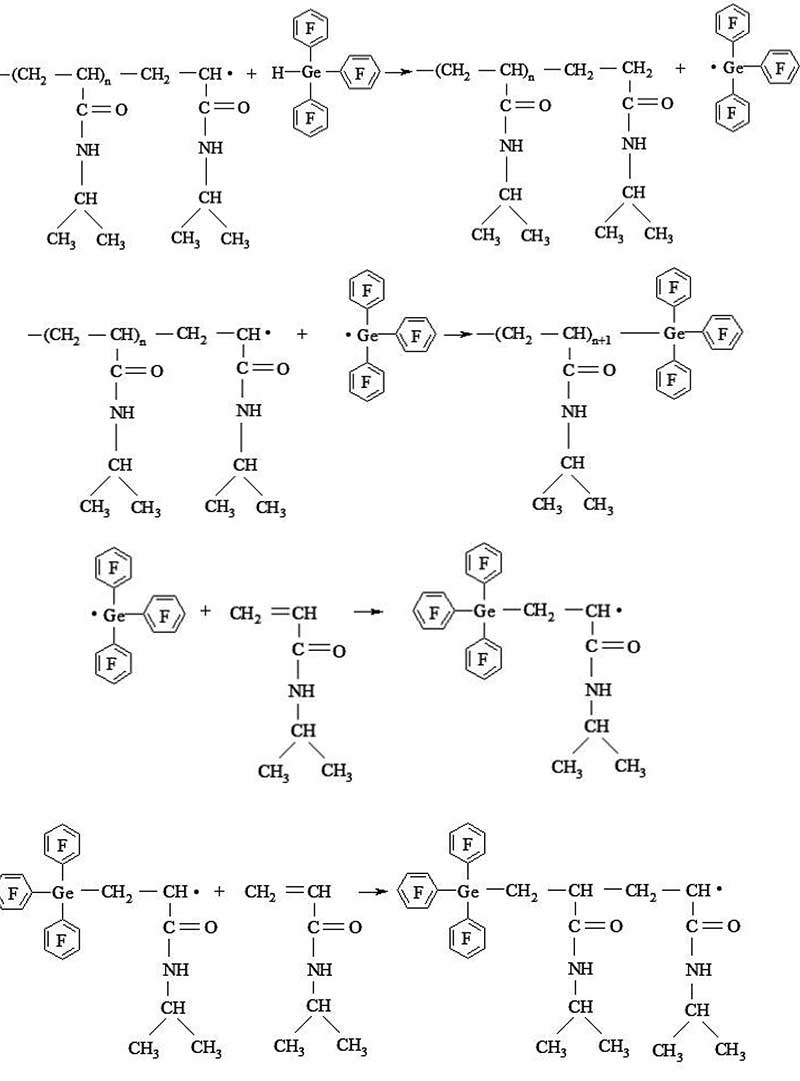
Figure 1. Chain transfer reaction to (С6F5)3GeH during radical polymerization of NIAA.
In Figure 1 shows the chain transfer reaction at (С6F5)3GeH during the radical polymerization of NIAA. The resulting functional polymer has an active group at the chain end, which can participate in the reaction of activated polycondensation with tris-(pentafluorophenyl) germane. Therefore, the functional polymer obtained on the basis of NIAA and(С6F5)3GeH (with (C6F5)3GeН = 0.02 mol/l) was used as a “seed” in the synthesis of the linear-dendritic block copolymer PNIAA‒PPG.
In Figure 2 shows the IR spectra of PNIAA (Fig. 2a), a functional polymer (Fig. 2b) containing a tris-(pentafluorophenyl)germane group at the end of the chain, and a block copolymer of PNIAA‒PPG (Fig. 2c).
From the data in fig. 2 that the absorption bands corresponding to the –−C6F5 (972.7 cm-1) and –CF (1082.7 cm-1) groups appear in the spectrum of the functional polymer, which confirms the presence of pentafluorophenylgermane groups at the end of the chain in the functional polymer molecules.
In the case of the block copolymer PNIAA‒PPG, absorption bands appear (1236.5 cm-1 and 947.3 cm-1), corresponding to the −С6F4 group, which argues in favor of the formation of a hyperbranched block at the end of the macromolecule.
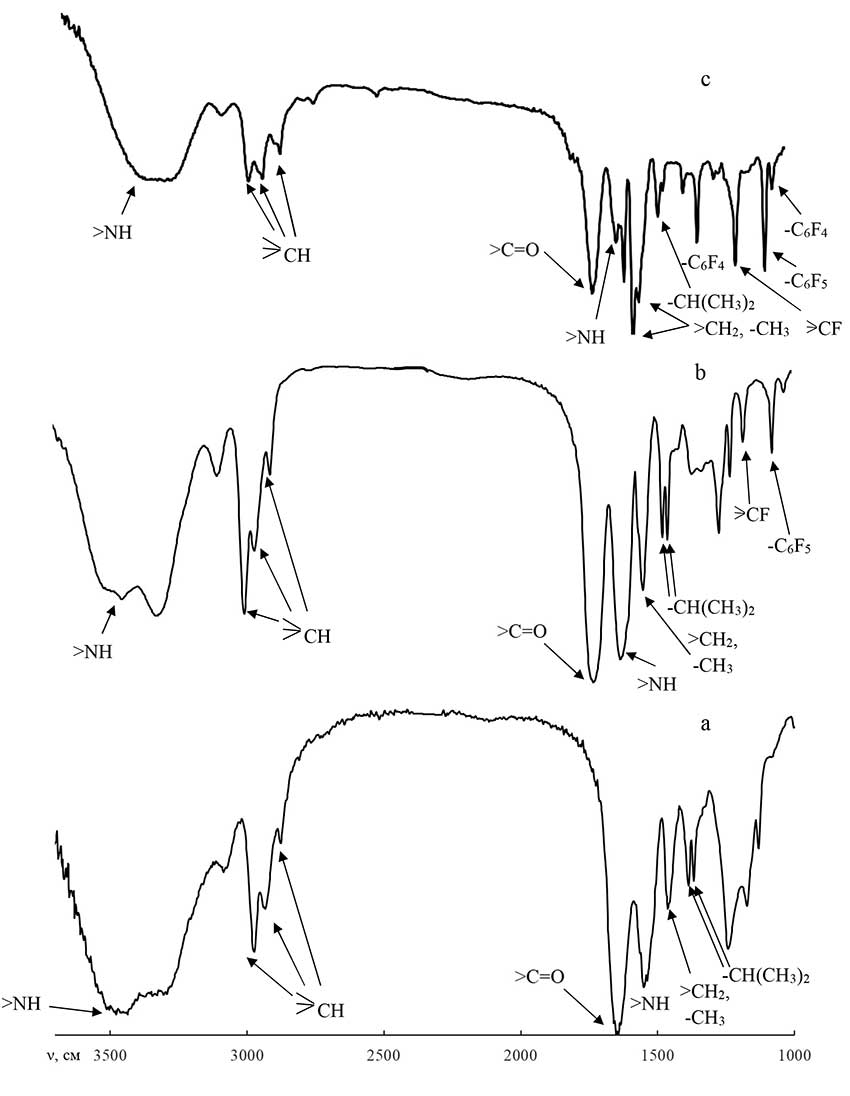
Figure 2. IR spectra of the homopolymer PNIAA (a), the functional polymer PNIAA‒Ge(С6F5)3 (b) and the block copolymer PNIAA‒PPG (c).
The functional polymer PNIAA‒Ge(С6F5)3 and the block copolymer PNIAA‒PPG were characterized by NMR spectroscopy. In fig. 3 and 4 are the 19F NMR spectra of PNIAA‒Ge(С6F5)3 and PNIAA‒PPG, respectively. On the spectrum of the block copolymer appears chemical. the shift of fluorine atoms of the group ‒С6F4 (-126.53 ppm), in addition, chemical shift of the fluorine atom of the ‒C6F5 group shifts to a higher region, which also indicates the formation of a hyperbranched block at the end of the macromolecules.
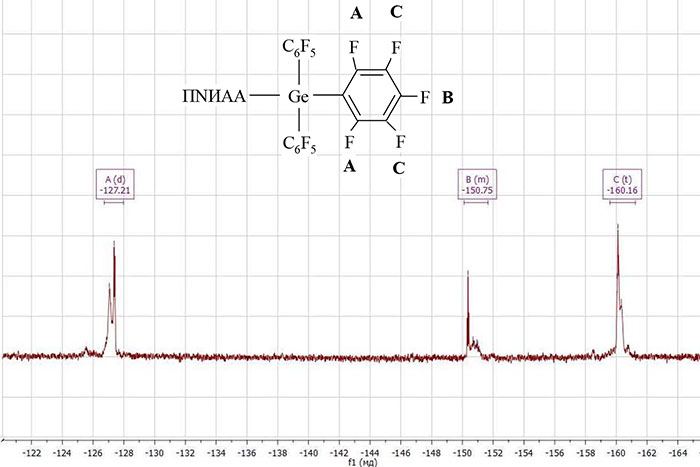
Figure 3. 19F NMR spectra of PNIAA‒Ge(С6F5)3.
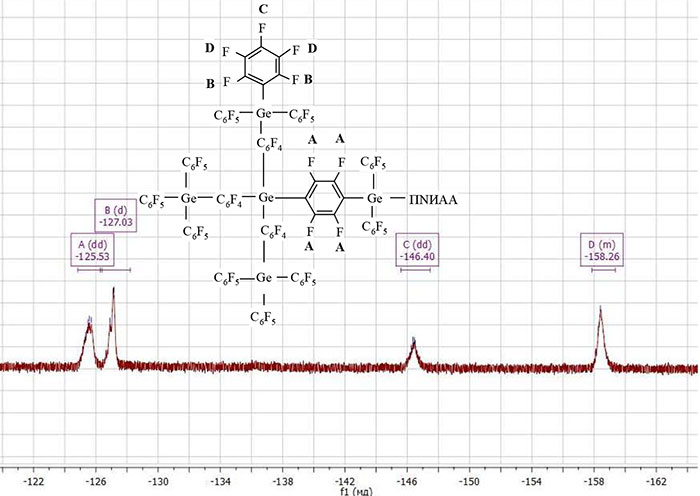
Figure 4. 19F NMR spectra of PNIAA‒PPG.
A diagram of the formation of a hyperbranched block at the end of the PNIAA chain is shown in Figure 5. This reaction is similar to the formation of hyperbranched PPG and proceeds in two stages [13]. At the first stage, monomer (С6F5)3GeH is activated, and at the second stage, step polymerization occurs, leading to the growth of the hyperbranched PPG macromolecule.
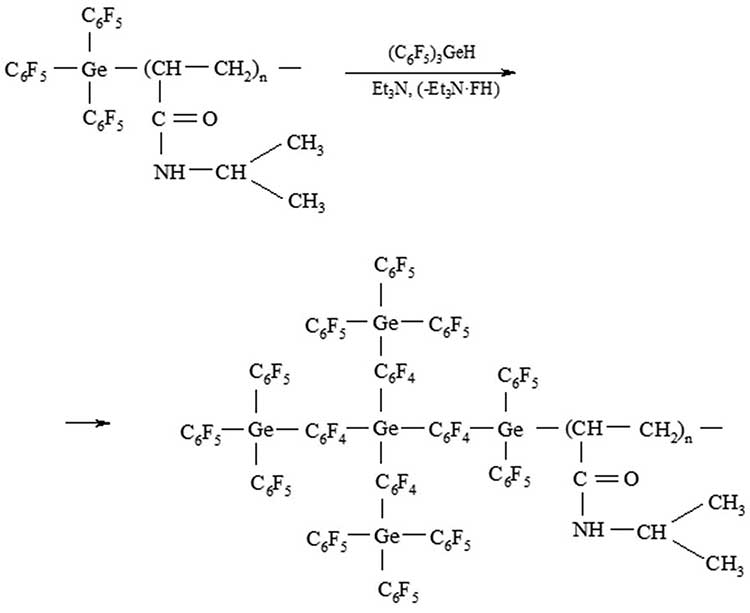
Figure 5. Scheme of formation of a block copolymer PNIAA‒PPG
The yield of the obtained block copolymer PNIAA‒PPG (21%) is significantly lower than with the activated polycondensation PNIAA‒Ge(C6F5)2H with tris-(pentafluorophenyl) germane (57%) [7]. This may be due to the fact that in this case only one germane group from (С6F5)3GeH is activated due to the formation of a donor – acceptor bond with Et3N. Accordingly, with the use of PNIAA‒Ge(C6F5)2H, another germane group appears, which can also participate in the reaction of activated polycondensation and the process will proceed with a high yield and speed. In addition, the formation of hyperbranched block affects the penetration rate (С6F5)3GeH in the macro club.
It has been established [8] that an increase in the molecular mass of the flexible chain polymer leads to a change in the conformation of the macromolecule, in which the functional group necessary for the reaction is located on the periphery of the flexible chain unit. At the same time, the reaction of activated polycondensation proceeds with great yield and speed. The difficulty of the reaction may be associated with steric hindrances of the para-C‑F positions in the C6F5 groups, which are centers of nucleophilic attack [14].
Thus, the possibility of a chain transfer reaction to tris-(pentafluorophenyl)germane during the radical polymerization of N-isopropylacrylamide has been established, thereby obtaining an amphiphilic linear-dendritic block copolymer based on N-isopropylacrylamide and tris-(pentafluorophenyl)germane. Tris- (pentafluorophenyl)germane proved to be an active chain transmitter in the polymerization of NIAA, the chain transfer efficiency was higher than in the polymerization of (С6F5)3GeH with MMA, N-VP and FMA (Table 1). An abnormally high Cs value during polymerization with styrene can be associated with a specific mechanism of breaking the material chain, due to the simultaneous course of cationic and radical polymerization [14]. If we compare the effectiveness of (С6F5)3GeH and (С6F5)2GeH2 as chain transmitters, then we can say that in the studied systems (Table 1) bis-(pentafluorophenyl)germane is more active.
Literature
- Gorelov A. V. Fiziko-khimicheskiye svoystva biologicheski znachimykh termochuvstvitelnykh polimerov. Diss. kand. fiz-mat. nauk. Pushchino, Institute of Theoretical and Experimental Biophysics RAS, 2008, 103 p. (in Russian).
- Selezneva I. I. Razrabotka i issledovaniye termochuvstvitelnykh materialov na osnove poli-N-izopropilakrilamida Diss. kand. fiz-mat. nauk . Pushchino, Institute of Theoretical and Experimental Biophysics RAS, 2004, 140 p. (in Russian).
- Zakharova O. G., Tarasova E. V., Simonova M. A., Semchikov Yu. D., Filippov A. P. Synthesis and structural and conformational properties of hybrid polymers of styrene with perfluorinated compounds of germanium // Polymer Science, Series A. 2009, 51(5), p. 512-517 .
- Simonova M. A., Khayrullin A. R., Zamyshlyayeva O. G., Filipov A. P. Svoystva gibridnykh lineyno-dendritnykh blok-sopolimerov lineynogo polimetilmetakrilata so sverkhrazvetvlennym polifenilengermanom v rastvorakh i plenkakh // Vestnik Tverskogo Gosudarstvennogo universiteta Seriya Khimiya. 2016, 2, p. 21-27 (in Russian).
- Simonova M. A., Zamyshlyayeva O. G., Simonova A. A., Filippov A. P., Semchikov Yu.D. Svoystva gibridnykh lineyno-dendritnykh blok-sopolimerov lineynogo polimetilmetakrilata so sverkhrazvetvlennym polifenilengermanom // Fiziko-khimiya polimerov: sintez, svoystva i primeneniye. 2014, 20, p. 182-187 (in Russian).
- Simonova, M. A. Zamyshlyayeva, O. G. Filippov, A. P. Simonova, A. A. Conformation of the linear-dendritic block-copolymers poly(methylmethacrylate) with hyperbranched polyphenylengermane // Int. J. Polym. Anal. Charact. 2015, 20, p. 223-230.
- Zamyshlyaeva O. G., Smirnov E. A., Zakharycheva N. S. Linear-dendritic block copolymers based on N-isopropylacrylamide and perfluorinated polyphenylengermanes: synthesis and properties at various interfacial boundaries // Polymer Science, Series B. 2017, 59(6), p. 708-717.
- Zamyshlyaeva O. G. (So)polimery razlichnoy arkhitektury na osnove perftorirovannykh gidridov germaniya: sintez, struktura i svoystva. Dis. dokt. khim. nauk . N. Novgorod, Lobachevsky State University of Nizhni Novgorod, 2013, 245 p. (in Russian).
- Vaysberger A., Proskauer E., Riddik D., Tups E. Organicheskiye rastvoriteli [Organic solvents]. Moscow, Foreign Languages Publishing House, 1958, 520 p. (in Russian).
- Fujishige S. Intrinsic viscosity-molecular weight relationships for PNIPAA solutions // Polymer Journal. 1987, 19, p. 297-300.
- Maya F. R. Chain Transfer in the Polymerization of Styrene: The Reaction of Solvents with Free Radicals // J. Am. Chem. Soc. 1943, 65, p. 2324-2328.
- Bochkarev M. N., Silkin V. B., Mayorova L. P., Razuvayev G. A., Semchikov Yu.D., Sherstyanykh V.I. Polyphenylenegerman - polymer material of a new type // Organometallic Chemistry in the USSR. 1988, 1, p. 108-112.
- Bochkarev M. N. Perfluorinated star-branched polymer // Polymer Science, Series B. 1989, 31, p. 643-644.
- Semchikov Yu. D., Zaitsev S. D., Katkova M. A., Bochkarev M. N., Zhernenkov M. N. Hybrid hyperbranched polymer based on polystyrene and tris-(pentafluorophenyl) germanium // Polymer Science, Series A. 2001, 43, p. 900-906.
ARTICLE INFO
Received 10 April 2019
Accepted 18 June 2019
Available online August 2019
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2019, 125, 7-8
