Received: October 2019
DOI 10.17677/fn20714807.2019.05.03
Fluorine Notes, 2019, 126, 5-6
EFFECT OF ELIMINATED RADICAL MASS AND MOLECULAR WEIGHT OF HOMOLOGS OF ALKYL HALIDES, α,ω–DIHALOALKANES,AND α,ω–DIBROMOPERFLUOROALKANES ON THEIR FRAGMENTATION UPON ELECTRON IMPACT IONIZATION
N. D. Kagramanov*1 and A. A. Tyutyunov1,2
1Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,Vavilova st.,28, Moscow, 119991 Russia
2P&M-Invest Scientific Production Association, Ltd. , Vavilova st., 28, Moscow, 119991 Russia
*e-mail: ndkagram@gmail.com
Abstract: Effect of eliminated radical mass and molecular weight of alkyl chlorides С4-С27 and alkyl bromides С4-С34 on the formation, growth, intensity drop, and transformation of cyclic onium ions C4H8Hal+ (1) and C5H10Hal+ (2) (where Hal = Cl, Br) has been studied for the series of 11–14 halogen-containing linear ions that differ by methylene groups. In the spectra of α,ω-dibromoalkanes C16–C20, the elimination of one of terminal bromine atoms produces a series of linear bromine-containing ions whose peak intensity is three times as high as the peak intensity of this series in the mass spectra of bromoalkanes. The elimination of one of iodine atoms in 1,10-diiododecane C10H20I2, according to mass spectra, leads to a series of peaks of linear iodine-containing ions, including C4H8I+ (30%) and C5H10I+ (25%), which are absent in the spectra of alkyl iodides. In the mass spectra of alkanes and perfluoroalkanes, bromine atom stabilizes C4H8 and CF2 groups with close masses. Change in fragmentation and ion peak intensity upon the growth of molecular weight of homologs results from the corresponding decrease of M+. excess energy. In the spectra of α,ω-dihaloalkanes, the elimination of terminal halide ion leads to decrease of excess energy fraction of M+. excitation, which is proportional to the mass of detached halide and intensity growth for the peaks of halogen-containing ions.
Keywords: halonium ions; alkyl halides; mass spectrometry; alkyl halide, α,ω-dihaloalkane, and α,ω-dibromoperfluoroalkane homologs fragmentation; dependence of fragmentation on homolog mass; dependence of ion peak intensity on the ratio of eliminated radical mass to ion mass.
Introduction
In this work, by the example of mass spectra of alkyl halides, we have studied the effect of homolog molecular weight and eliminated radical mass on the formation, growth, intensity drop, and transformation of cyclic onium ions C4H8Hal+ (1) and C5H10Hal+ (2) (where Hal = Cl, Br) in the series of 11–14 halogen-containing ions of linear structure that differ by methylene group number. The cyclic five-membered structure of stable onium ions 1 postulated in the work [1] was confirmed experimentally by the collision activation spectra of ions ХC35Cl+ (where X = 2H or 2D or HD), produced from 1-chlorohexane, 1-chlorohexane-1,1-d2 , and 1-chlorohexane-4,4-d2, on extended H/D scrambling [2].
Iodine inefficiency in the formation of ions 1 was explained [1, 2] by its relatively poor ability to accept electron to form the second bond of onium ring. Reviews [3, 4] also reported that the spectra of alkyl iodides contain no ion 1. To reveal the reason of the lack of iodine-containing ions, we have analyzed the mass spectra of homologs of α,ω-diiodooalkanes C2–C20 from the NIST libraries.
Another task of the study was to elucidate the dependence of peak intensity for chlorine- and bromine-containing onium ions 1 and 2 on homolog molecular weights and the reasons of their formation completion. To solve this task, we analyzed the mass spectra of primary alkyls with terminal Cl and Br atoms from the NIST libraries and performed a retrospective review of spectra reported in the work [1]. We studied the changes of fragmentation, intensities, and the number of peaks of halogen-containing ions observed on the growth of homolog molecular weight and the dependence of 1 intensity on the ratio of ion 1 mass to the mass of eliminated radical [M-1].
Molecular weight is a macroscopic property that includes elemental composition, molecule structure, and bond energies, nonetheless, in the context of linear alkyl halide homologs, whose structures and bond energies are constant, increase in molecular weight should lead to decrease of the vibrational excitation of M+.and the corresponding change in fragmentation.
There are no contemporary studies on the effect of homolog molecular weight on the fragmentation and intensity of resulting typical base peaks except for the MS/MS study of peptide fragmentation where the authors [5] found the effect of peptide mass on the growth and decrease of the number of ions and peak intensity for the series of two different types: -y and -b, resulting from peptide chain cleavage.
The order of alkyl substituents elimination in N-(tert-alkyl)amines and -acetamides in accordance with their mass growth and the enhancement of peak intensity for ions formed upon increase in the weight of eliminated alkyl radicals was described as "ion mass effect" and quantitatively calculated by Za’horszky [6]. In domestic reviews on mass spectrometry, this effect is called "the rule of elimination of the maximal radical" [7]. Seemingly, there are no examples for the use of this rule when eliminated radical is heavier than the resulting ion.
The first studies of homologs fragmentation were performed for n-alkanes [8]. They dealt with the role of different formation mechanisms for [C3H7]+ ions and the main decomposition schemes confirmed by metastable peaks [9]. The effect of homolog molecular weight on the fragmentation of normal paraffin hydrocarbons was not considered in the works [8, 9].
The mass spectra of completely fluorinated n-alkanes С2–С6 were studied to compare their fragmentation with hydrocarbon analogs [10] and to determine their ionization potentials. In the series of perfluoroalkanes С2–С6, PI1 decreases upon chain growth from 14.48 to 12.74 eV, respectively [11].
Experimental
The samples of α,ω-dibromoperfluoroalkanes C4–C10, whose spectra are absent in the NIST libraries, were presented by the P&M-Invest Scientific Production Association, Ltd.
Their EI mass spectra were registered with a VG-7070E mass spectrometer in the mass range 30–700 Da at ionizing voltage 70 eV (Rtx-5MS capillary column, 5% diphenyl/95% dimethylpolysiloxane, 30 m, 0.25 mm internal diameter, max. temperature 350C).
Mass spectra of alkyl chlorides and alkyl bromides
In the spectra of alkyl chlorides and alkyl bromides, the peak of onium ion 1 remains basic until the mass of alkyl radical eliminated upon its formation becomes larger than 1 mass (91 Da for chlorides and 135 Da for bromides). Four fragmentation processes alternate upon increase in molecular weight and decrease of vibrational excitation of M+.:
(1) HCl abstraction in C2–C5 or the corresponding Br abstraction in C1–C6;
(2) formation of ions 1 and 2 upon abstraction of alkyl radicals with masses lower 1 and 2 in the spectra of C6-C10 chlorides and C7-C14 bromides
(3) decrease of 1 intensity upon abstraction of radicals with masses larger than 1, increase in M+. intensity and formation of a series of halogen-containing ions different by the number of methylene groups with masses larger than 2, resulting from the abstraction of radicals with masses lower than 2 in the spectra of C11–C18 chlorides and C15–C20 bromides;
(4) intensity decrease for all halogen-containing ions owing to energy shortage on account of redistribution of increasing portion of М+ energy for alkyl chain elongated as compared with 1 and 2. Prevailing formation of a series of intense alkyl ions with base C4H9+ ion in the spectra of C27 chlorides and C34 bromides takes place.
We considered in detail the effect of homolog molecular weight on onium ions formation by the example of alkyl chlorides.
We should emphasize the complete coincidence of spectra represented in this paper in graphical format with the tabular spectra [1]. The author [1] did not discuss the reasons of intensity decrease of ion 1 peak in tables from 100% to 32% for chloroalkanes and from 100% to 54% for bromoalkanes when molecular weight increases by 84 Da. The series of halogen-containing ions from molecular ion to ion 2 present in tabulated mass spectra [1] were either missed or ignored.
The plots for the main peak intensities in the mass spectra of alkyl chlorides C1–С27 (Fig. 1) display their dependence on molecular weight within 50–414 Da. Only C1–С2 homologs have 100% peak intensity of molecular ion because fragmentation in C1–С2 is minimal as compared with higher homologs.
The intensity of M+. in C3–С6 spectra decreases to 0% and retains at this level up to C12, until base ion 1 formation and its intensity drop begins. The further growth of molecular weight (C14–С22) leads to increase in M+. from 1% to 5% and formation of a series of chlorine-containing ions (Figs. 2 and 3) preceding to ions 2 and 1.
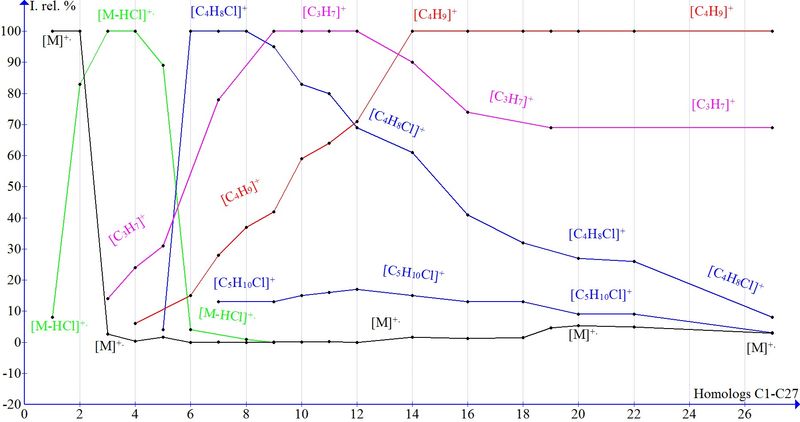
Fig. 1. Dependence of Irel. (%) of the ion peaks of alkyl chlorides on homolog molecular weight.
Alkyl chlorides C1–C4 (m/z 50/52 to m/z 92/94) undergo fragmentation with abstraction of .Cl and HCl to give intense and base ions [CH3]+, [C2H5]+, [C3H6]+, [C4H8]+.
In the mass spectrum of C5 homolog (m/z 106/108), the elimination of HCl (89%) is continued but ion 1 (m/z 91/93) with peak intensity of 4% appears due to methyl radical elimination. When molecular weight increases, the growth of ion 1 peak intensity up to 100% in C6–C9 due to decrease of HCl elimination to 0% is accompanied by the growth of peak intensity for alkyl ion [C3H7]+ (m/z 43 (72–100%) resulting from elimination of chloroalkyl radicals.
The second ion 2 (m/z105/107) with peak intensity 13% appears only in С7 spectrum. The third chlorine-containing ion [C6H12Hal]+ 3 (m/z 119/121); 0.5%, appears in С8 spectrum, while its peak intensity in C9 spectrum increases to 1%, and the fourth ion [C7H14Hal]+ 4 (m/z 133/135), 0.3%, appears.
Further increase in molecular weight leads in the spectrum of C14 (m/z 232/234) to decrease of peak intensity for 1 to 61%, and increase in peak intensity for ions 2 (m/z 105/107), 3 (m/z 119/121), and 4 (m/z 133/135) to 15% , 4%, and 2.3%, respectively, and emergence of the fifth ion [C8H16Hal]+ 5 (m/z 147/149), 1.4%, and the sixth ion [C9H18Hal]+ 6 (m/z 161/163), 0.8%.
The plot of peak intensity for chlorine-containing ions of alkyl chlorides (Fig. 2) displays their emergence, growth, and decrease dynamics as homolog molecular weight rises.
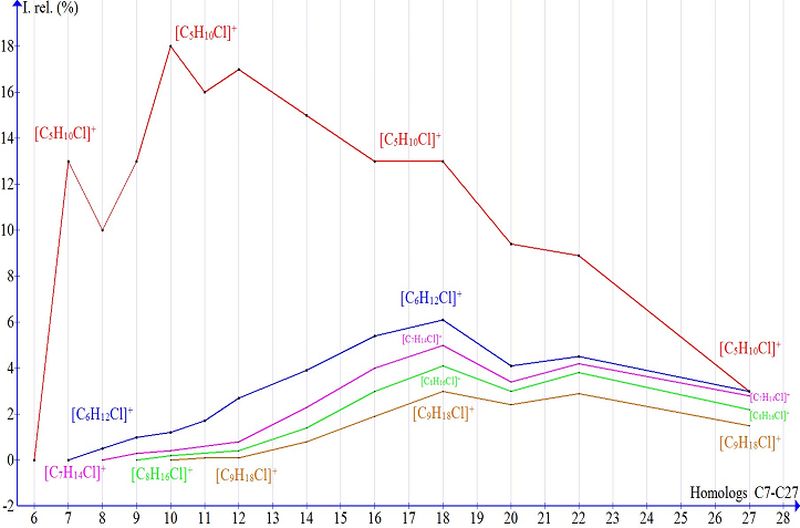
Fig. 2. Peak intensity of chlorine-containing ions in the spectra of C7–C27 alkyl chlorides.
Figure 2 shows the lack of the most intense ion 1 because its presence makes difficult to consider less intense ions. The mass spectrum of homolog C18 (m/z 288/300) with peak intensities of ions 1 (32%), 2 (13%), and 3 (6%) contains a series of 11 chlorine-containing ions of alkyl chlorides with masses >1 and 2 (Fig. 3); they result from the elimination of radicals with lower masses than that upon formation of ion 1.
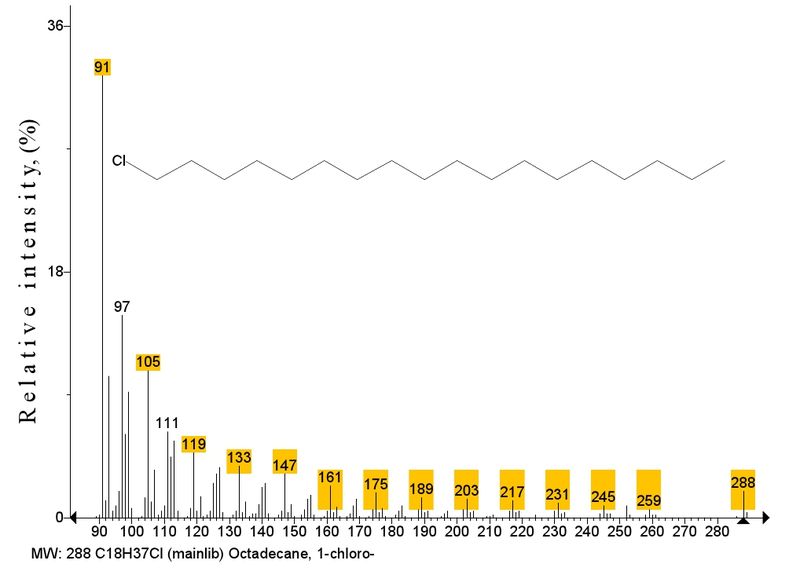
Fig. 3. A series of chlorine-containing ions in the spectrum of octadecyl chloride.
The growth of molecular ion and emergence of ion series (Fig. 3) in the spectrum of homolog C18 results from increase in molecular weight and the corresponding drop of internal energy of M+ as compared with homologs with lower molecular weights. According to the quasiequilibrium theory of monomolecular reactions [12], excitation energy over time 10-12 s is uniformly distributed over all bonds of molecule. Assuming that the energy of M+ is distributed between ion 1 and radical [M-1] in proportion to their masses, the excess of radical mass over the ion mass should result in decrease of the share of energy necessary for ion formation and decrease of its intensity. Peak intensity of 1 92% in the spectrum of C10 corresponds to the ratio of ion 1 mass (91 Da) and eliminated upon its formation alkyl radical (85 Da) of 91/85=1.07. In the spectrum of C18 where ion 1 peak intensity is 32%, mass ratio of ion 1 and eliminated upon its formation radical C14H29 (197 Da) is 91/197 = 0.46. The formation and intensity growth of 1 proceeds when eliminated radical mass is lower than or equal to the mass of ion 1 (91 Da).
Ion 1 of 91 Da resulting from the decomposition of M+.of С18 due to enhanced to 197 mass of eliminated radical as compared with С10 shows "energy deficiency" and decreased intensity as compared with situation when the mass of eliminated radical was equal or lower than ion mass. The energy deficiency due to molecular weight growth probably results in less energy-spending fragmentation processes of radical elimination with masses lower than 2 to form a series of new halogen-containing ions with masses higher than 2. The series of 14 chlorine-containing ions different by the number of CH2 groups in the spectrum of С18 (Fig. 3) allows one to draw a conclusion on their similar linear structure.
It should be noted that the peak intensities of ion series decrease in proportion to the growth of their masses.
The further growth of molecular weight leads in the spectrum of C22 to decrease of peak intensity to 26%, 9%, and 4.5% for ions 1, 2, and 3, respectively. The intensity of 1 in the spectrum of С27 is 8%, and a series of alkyl ions [CnH2n+1]+ becomes predominant. An additional reason to terminate chloroalkyl fragmentation may be that the mass of its base ion 1 is by factor of 1.6 larger than the mass of alkyl base ion C4H8 of 57 Da. The difference in the masses of base ions for two competitive fragmentations may provide additional preference for alkyl fragmentation.
Mass spectra of α,ω-dibromoalkanes
As compared with alkyl halides, fragmentation processes occurred in α,ω-dihaloalkanes are of interest to elucidate the effect of the second terminal halogen atom on the intensity and number of formed halogen-containing ions and the formation of alkyl ions [CnH2n+1 ]+ in the absence of terminal methyl group.
The fragmentation changes in the mass spectra of α,ω-dibromoalkanes С2–C32 were considered when molecular weight increased to 186–606 Da.
The mass spectra of homologs C2–C7 show both simultaneous elimination of Br radical and HBr molecule to give intense alkenyl ions [CnH2n-1]+ and elimination of one of bromine atoms to form bromine-containing ions.
When molecular weight of C2–C7 rises, the intensity of [M-Br]+ peak decreases from 100% to 8 % and the spectrum shows the emergence of ion 1 of 16%.
Process [M+.– (.Br+HBr)] excludes the formation of stable bromine-containing ions and alkenyl ions [CnH2n+1]+: [C2H3]+, [C3H5]+, [C4H7]+, [C5H9]+, [C6H11]+ , and [C7H13]+ with increasing masses from 27 to 97 become base ions in the spectra of C2-C7.
In the mass spectrum of C8 (m/z 270/272), [M-(Br+HBr)]+ ion peak intensity decreases to 53%, [C5H9]+ becomes base ion, while ion 1 intensity increases to 29% and retains at this level also for C14 homolog due to decomposition of [M]+. / 2. However, [M/2]+ ion intensity of 29% is maximal only for homolog C8, because increase in homolog mass upon molecule cleavage into halves leads to formation another pair of ion and radical with higher mass and lower ion peak intensity.
Ion 2 (m/z 149/151) 8% also appears in the spectrum of C8.
The number of peaks of bromine-containing ions increases in the mass spectra of С9-С20 homologs. The spectrum of C16 (m/z 382/384) contains 11 peaks, the spectrum of C20 (m/z 438/440) (Fig. 4) the series rises to 14 bromine-containing ions that differ by CH2 groups, which enables one to draw a conclusion on their similar linear structure.
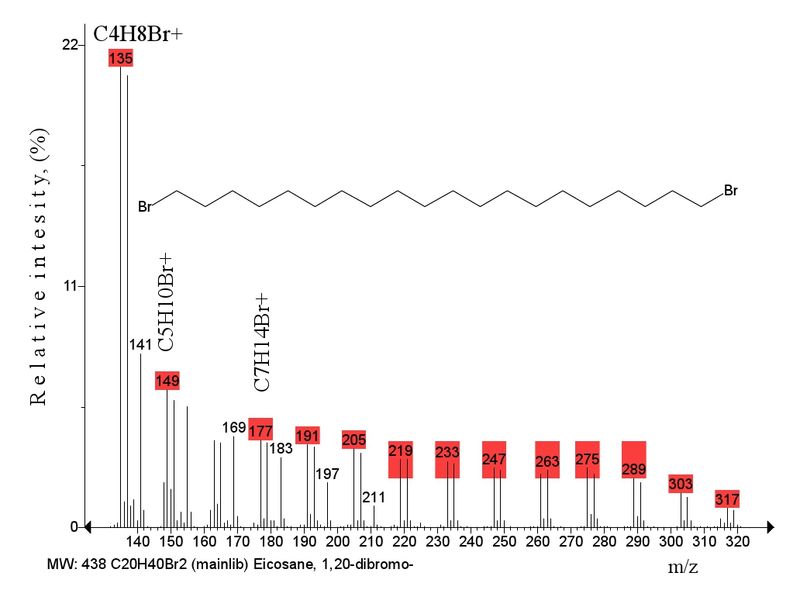
Fig. 4. The series of bromine-containing ions in the mass spectrum of C20H40Br2.
The mass spectra of α,ω-dibromoalkanes C9-C20 (m/z 284/286 – 438/440) retain peak 1 intensity at the level of 27%-22%, i.e., in comparison of alkyl bromide homologs where increase in molecular weight leads to a rather sharp decreases of 1 intensity, the intensity of bromine-containing ions decreases less sharply when chain length of α,ω-dibromoalkanes rises. In the spectra of C9-C12, [C4H7]+ becomes base ion. In spite of hindered alkyl fragmentation in α,ω-dibromoalkanes as compared with alkyl bromides, it begins simultaneously with the growth of peak 1 intensity. Peak intensity of [C4H9]+ ion, which belongs only to rearranged ion, reaches 100% in C14 spectrum and retains at this level.
As compared with mass spectra of alkyl bromides where the maximal intensity of ion 1 is 100%, the spectra of α,ω-dibromoalkanes show ion 1 intensity not higher 30% due to additional channel of [M+.-(.Br+HBr)] elimination, whereas the intensities of bromine-containing ions 3, 4, and all series is almost three times larger because of decrease of energy for resultant [C20H40Br]+ ion due to elimination of bromine radical.
Upon further chain elongation, the spectrum of C32 shows decrease of peak 1 intensity to 7%, the intensity of [M-Br]+ also decreases to 15%, peak intensity for the series of bromine-containing ions decreases and the series of alkyl and alkenyl ions become predominant.
Mass spectra of α,ω-dibromoperfluoroalkanes
The mass spectra of α,ω-dibromoalkanes and α,ω-dibromoperfluoroalkanes allow one to compare the stabilizing ability of bromine atom mass upon formation of bromine-containing ions in alkyl and perfluoroalkyl chain.
The mass spectra of decafluorobutane (A), nonafluoro-1-bromobutane (B), and α,ω-dibromoperfluorobutane (C) presented in Fig. 5 illustrate fragmentation changes on introduction of one or two terminal bromine atoms in molecule. Spectrum B shows a peak of ion [M-Br]+ (m/ z 219) 66% and its fragmentary ions [C2F5]+ and [C2F4]+, whose intensities as compared with spectrum A is by more than twice as large. The peak intensity of allyl ion [C3F5]+ (m/ z 131) remains the same as in the spectrum A taking into account its intensity as a difference between intensities of isotope ions 131 and 129 (66% – 70% = 7%).
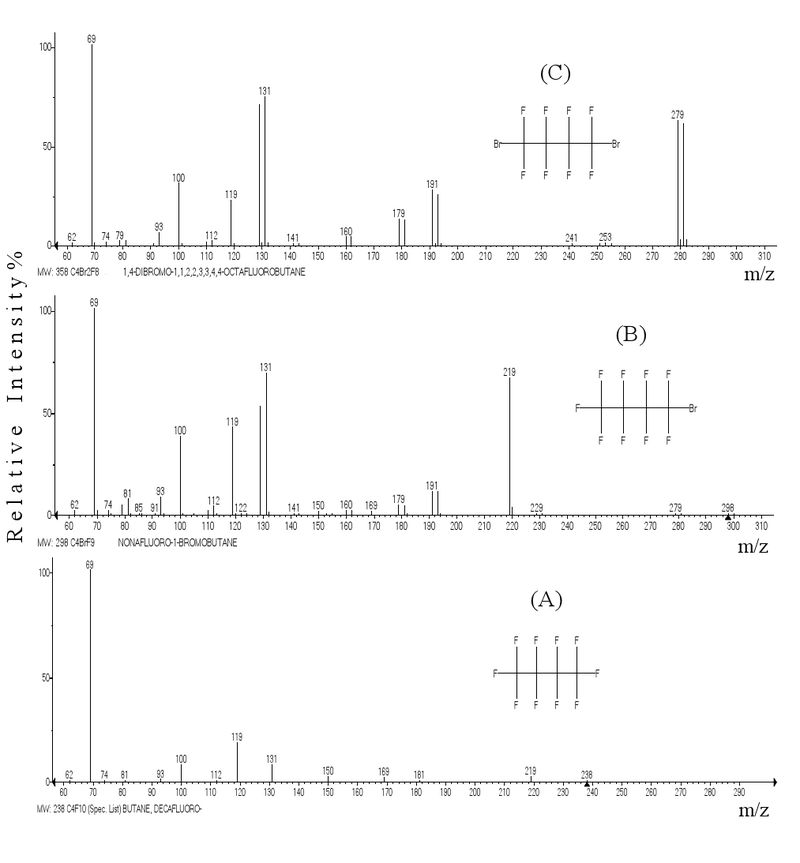
Fig. 5. Mass spectra of decafluorobutane (A), nonafluoro-1-bromobutane (B), and 1,4-dibromooctafluorobutane (C).
The spectrum B shows a peak of ion [C4F9]+ (m/ z 219) 66% resulting from elimination of Br and the peaks of bromine-containing ions [BrCF2]+ (m/ z 129) 52%, [BrC2F4]+ (m/ z 179) 5% and alkenyl ions [BrC3F4]+ (m/ z 191) 11% and [BrC2F3]+ (m/ z 160) 2%.
The spectrum C exhibits a strong peak [BrC4F8]+ (m/ z 279) 62% due to Br elimination. As compared with the spectrum B, the peak intensities of bromine-containing ions [BrCF2]+ (m/ z 129) 70% and [BrC2F4]+ M/2 (m/ z 179) 13% increases by a factor of 1.4–2. The peak intensities of alkenyl ions [BrC3F4]+ (m/ z 191) 27% and [BrC2F3]+ (m/ z 160) 4% as compared with the spectrum C increase by more than twice, while the intensity of perfluoroallyl ion [C3F5]+ (m/ z 131) decreases by the same factor.
The plots of peak intensities for the main ions in the mass spectra of α,ω-dibromoperfluoroalkanes С2–C10 (Fig. 6) show their dependences upon the growth of molecular weight within 258–658 Da and perfluoroalkyl chain mass within 100–500.
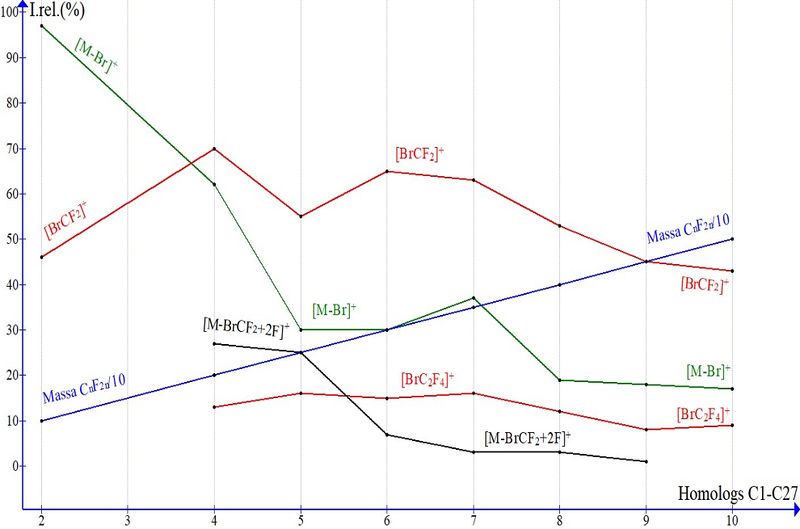
Fig. 6. Dependences of Irel % of perfluoroalkyl dibromides on homolog molecular weight.
The fivefold increase in molecular weight of perfluoroalkyl chain leads to the fivefold decrease of [M-Br]+ peak intensity. The revealed dependence is explained by the fact that elimination of bromine radical upon increase in chain weight causes decrease of vibrational excitation energy of M+..
Due to the closest proximity of two terminal BrCF2 groups, the decomposition of molecular cation radical C2 leads to intense peak [BrCF2]+ 46% and the peaks of rearrangement ions: [CF3]+ of medium intensity,18%, with mass 69 and weak 4% peak [CFBr2]+ with mass 189, which indicates the relationship between peak intensity for these ions and their masses.
In the spectra of α,ω-dibromoperfluoroalkanes, the peak intensity of [M/2]+ ion decreases from 46% for C2 to 0% for C8, Decrease in peak intensity for ions [BrCF2]+ and [BrC2F4]+ is less dramatic because it is equilibrated by their minimal masses and maximal stabilizing ability of bromine atom mass.
Thus, we can conclude that the maximal chain mass stabilized by bromine atom in the mass spectra of alkyl bromides and α,ω-dibromoalkanes is the same (56 Da in alkanes and 50 Da in perfluoroalkanes. The transformation of cyclic onium ions 1 and 2 in the series of ions of linear structure allows us to conclude that stabilization of alkyl group by halogen atom due to absorption of excess vibrational energy is caused not only by ion structure. It appears when Hal atom mass is larger or close to the mass of stabilized group. The efficiency of such stabilization is dependent not only on the ratio of masses of Hal atom and the stabilized group but also on bond strength.
Mass spectra of alkyl iodides, 1-iodo-11-bromoundecane, and α,ω-diiodoalkanes
The mass spectra of alkyl iodides C8H17I and C10H21I show the series of weak peaks of iodine-containing ions: [CH2I]+ (m/z 141) 5% and [C2H4I]+ (m/z 155) 5%, [C3H6I]+ (m/z 169) 0.5%, 1 (m/z 183) 0.5% and 2 (m/z 197) 0.1%.
In the spectrum of 1-iodo-11-bromoundecane C10H22BrI, the peak intensity of a series of iodine-containing ions increases due to the terminal bromine atom and peak 1 (m/z 183) rises to 9.2%, while the peak intensity of bromine-containing ion 1 (m/z 135) as compared with the spectrum of 1-bromoundecane C11H23Br decreases by more than 3 times from 100% to 29%. The presence of two different terminal halogen atoms leads to formation of two different series of halogen-containing ions and in this case to the amplification of peaks of weaker iodine-containing series and attenuation of peaks of more intense bromine-containing series.
The mass spectra of C2-C20 α,ω-diiodoalkanes (282–534 Da) show elimination of one of iodine atoms and simultaneous elimination I radical and HI molecule form molecular cation radical to give intense alkenyl ions.
Elimination [М+.– (.I+HI)] excludes the formation of stable iodine-containing ions, while the elimination of one iodine atom increases intensity of [M-I]+ peak in the spectrum of C5 up to 100 %. In the spectrum of C10 (m/ z 394), the intensity of this peak decreases to 37% but a series of 6 peaks of iodine-containing ions appears: 2 25%, 1 30%, and fragmentary ion [C2H4I]+(m/z 155) 50%, including. It is impossible to explain such a strong effect of terminal iodine atom elimination by only CH2-I bond dissociation energy, which is weaker than CH3-CH3 bond ΔH298 97.1 kcal mol-1 [13] because elimination of methyl or ethyl radical in alkyl iodides causes no significant changes in the intensity of iodine-containing ions. It seems to be taken into account not only bond cleavage but also elimination of mass of 127 Da with proportional to this mass excitation energy.
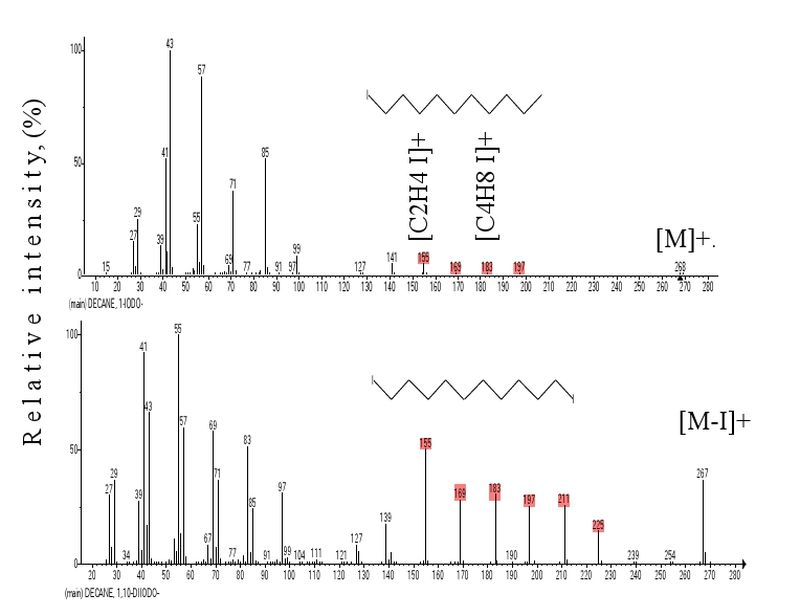
Fig. 7. Mass spectra of decyl iodide and 1,10-diiododecane.
The mass spectra of decyl iodide and 1,10-diiododecane presented in Fig. 7 display a tenfold increase in the intensity of iodine-containing ions in the spectrum of 1,10-diiododecane as compared with that of decyl iodide. A series of 6 iodine-containing ions different by methylene groups, ions 1 and 2 including (Fig. 7), allows one to conclude their similar linear structure. The lack of iodine-containing ions in the spectra of alkyl iodides is caused by too low energy of СH3-I bond, ΔH298 56.1 kcal mol-1 [13].
The larger intensity of [C2H4I]+ fragmentary ion peak as compared with peak 1 intensity results from the instability and easy decomposition of ion 1.
Because of elimination channel [M+.– (.I+HI)], the maximal intensity of 1 in the spectrum of C10H20I is not larger 30% and decreases to 20% in the spectrum of C20 (534 Da) due to twofold increase in homolog mass.
Acknowledgments
This work was performed with the financial support from Ministry of Science and Higher Education of the Russian Federation using the equipment of Center for molecular composition studies of INEOS RAS.
References
- McLafferty F. W. Analytical Chemistry, 1962, 34(1), 2-15.
- Van de Sande, McLafferty F.W. J. Am. Chem. Soc., 1975, 97, 2298-2299.
- Budzikiewicz H., Djerassi C., Williams D.H. Mass Spectrometry of Organic Compounds. San Francisco, Holden-Day Inc., 1967. 690 p.
- Vulfson N. S., Zaikin V. G., Mikaya A. I. Mass Spectrometry of Organic Compounds. Khimiya, Мoscow, 1986, p. 117.
- Sheila J. Barton and John C. Whittaker // Mass Spectrometry Reviews, 2009, 28, 177-187.
- U. I. Za’horszky, Oranic Mass Specrometry, 1979, 14(2), 66-75.
- Lebedev A.T. Mass Spectrometry in Organic Chemistry. Binom, Мoscow, 2003, pp. 44–46.
- Beynon J. H., Mass Spectrometry and Its Applications to Organic Chemistry, Mir, Мoscow, 1964, pp. 330–335.
- Meyerson S., J.Chem. Phys., 1965, 42, 2181.
- Majer J.R., “Mass spectrometry of fluorine compounds” in Storey M., Tatlow J.C. and Sharp A.G. (Eds), “Advances in Fluorine Chemistry”, Vol.2, Butterworth, Washington 1961, p.55 and the refereces therein.
- Robin M. B. Higher Excited States of Polyatomic Molecules.- N.Y.: Acad. Press.-1974.-V.1.-256p.
- Rosenshtock H.M., Wallenstein H.B., Warhafig A., Eyring H, Proc. Natl. Acad. Sci USA, 1952, 38, 667.
- Kandel. B.J. J. Chem. Phys., 1954, 22, 1496.
ARTICLE INFO
Received 02 October 2019
Accepted 14 October 2019
Available online October 2019
Recommended for publication by Prof. S. Igumnov
Fluorine Notes, 2019, 126, 5-6
