Received: December 2019
DOI 10.17677/fn20714807.2020.02.02
Fluorine Notes, 2020, 129, 3-4
STUDY OF INTERACTION OF COMPLEX CATALYST BF3·H2O WITH p-METHYLSTYRENE IN TOLUENE BY AB INITIO METHOD
V.A. Babkin1, D.S. Andreev1, A.V. Ignatov1, V.S. Belousova2, A.N. Liberovskaya1, A.V. Kozhukhova1, E.S. Titova3,4, A.I. Rakhimov3, N.A. Rakhimova3, V.T. Fomichev6
1Volgograd State Technical University (Sebryakovsky br.), 403343 Volgograd Region, Mikhailovka, Michurina st., 21
e-mail: babkin_v.a@mail.ru
21st Moscow State Medical University n.a. I.M. Sechenov, 119991, st. Trubetskaya, 8, bld. 2
3Volgograd State Technical University, 400005 Volgograd, Lenin av., 28,
e-mail: organic@vstu.ru
4Volgograd State Medical University, 400131 Pavshikh Bortsov sq., 1
5V. I. Razumovsky Saratov State Medical University, 410012 Saratov, Bolshaya Kazachya st., 112
6Volgograd State Technical University (Architecture and Construction Institute), 400005 Volgograd, Lenin ave., 28
e-mail: titova051@rambler.ru
Abstract: A quantum chemical study of cationic polymerization monomer protonation of p-methylstyrene (in the presence of complex catalyst - boron fluoride/water in toluene - with stoichiometric composition of mixture 1:1:1) by ab initio method was first performed. It was found that the activation energy of this reaction is 136 kJ/mol, and the thermal effect is 116 kJ/mol.
Keywords: n-methyl styrene, protonation, boron fluoride/ water catalyst, toluene, reaction heat, ab initio method.
Introduction
Until now, a number of important fundamental questions remain concerning the course of elementary
acts of cationic polymerization of p-methylstyrene in the presence of a complex catalyst
which is often used in practice as an indifferent solvent, remain [1]. Therefore, the object of this
work is to study the protonation procedure of p-methylstyrene in the presence of this complex
catalyst.
Methodology
The protonation of cationic polymerization monomer of p-methylstyrene in the presence of a complex catalyst BF3∙H2O in toluene (with a stoichiometric composition of the mixture 1:1:1, respectively), was studied. The distance between the C(1) and H(20) atoms was chosen as the reaction coordinate. The calculation was performed using ab initio RHF/6-311G** quantum-chemical method [2] with geometry optimization for all parameters by the gradient method integrated into Firefly program [3], based on GAMESS source code [2, 4]. This method was chosen because it allows you to accurately calculate the reaction energy barriers and active centers (AC) [4]. The calculations were performed within the framework of molecular model and isolated molecule in toluene according to technique described in detail in [5] and applied in [6-19]. For visual molecular representation the MacMolPlt program was used [10].
Calculation data
The changes in bond lengths along the interaction coordinate, valence angles and atomic charges of molecular system during protonation reaction of p-methylstyrene in the presence of BF3∙H2O catalyst in toluene (the stoichiometric composition 1: 1: 1) are presented in Tables 1-3. Можно либо ед, либо множеств, в анг. Тоже можно так и так
Fig. 1 presents the geometric and electronic structure of initial model of p-methylstyrene,
in Fig. 2 - the structure after interaction of catalyst with p-methylstyrene, Fig.
3 – the energy profile of this interaction, Fig. 4 – the changes in charges on atoms directly involved
in interaction of complex catalyst BF3·H2O with p-methylstyrene.
The atoms C(1), C(2), H(20), O(21) and B(23) are directly involved in this interaction. Let us analyze the change in charges on these atoms along the reaction coordinate.
At coordination stage (1st stage, steps 1-7), the charge on the C(1) atom changes from -0.219 to -0.254; at the stage of active center formation (2nd stage, steps 8-17) - from -0.266 to -0.358; at the stage of final product formation (3rd stage, steps 18-21) - from -0.361 to -0.262.
At 1st stage, the atomic charge on C (2) changes from -0,098 to -0,108; at 2nd stage - from 0,332 to 0,359; at 3rd stage - from -0,080 to 0,081.
At 1st stage, the atomic charge on H(20) changes from 0,325 to 0,329; at 2nd stage - from -0,104 to -0,100; at 3rd stage - from 0,360 to 0,213.
At 1st stage, the atomic charge on О(21) changes from -0,437 to -0,438; at 2nd stage - from -0,438 to -0,453; at 3rd stage - from -0,467 to -0,613.
At 1st stage, the atomic charge on B(23) changes from 0,820 to 0,818; at 2nd stage - from 0,819 to 0,794; at 3rd stage - from 0,790 to 0,809.
The charges on toluene atoms (C(27) -C(33) and H(33) -H(40)) along the reaction coordinate varied in the following ranges: for C(27) -C(33): from -0.176 to -0.119; for H(33)-H(40): from 0.139 to 0.123. During of reaction, the O(21)-H(20) bonds are simultaneously broken, the C(1) -C(2) changes from double π-bond to a single σ-bond, the new bond C(1) - H(20) and the counterion [BF3∙OH] is formed (Fig. 2).
The values of activation energy and thermal effect are calculated, equal to 136 kJ/mol and 116 kJ/mol, respectively.
Thus, the changes in atomic charges, behavior of reaction fragments, breaking and formation of new bonds in the reaction under study indicate that it proceeds according to the scheme of coordinated interactions.
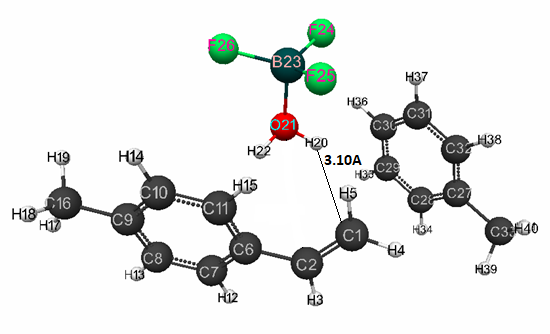
Figure 1. The structure of initial model of complex catalyst BF3·H2O in toluene (at stoichiometric composition 1:1:1) with p-methylstyrene.
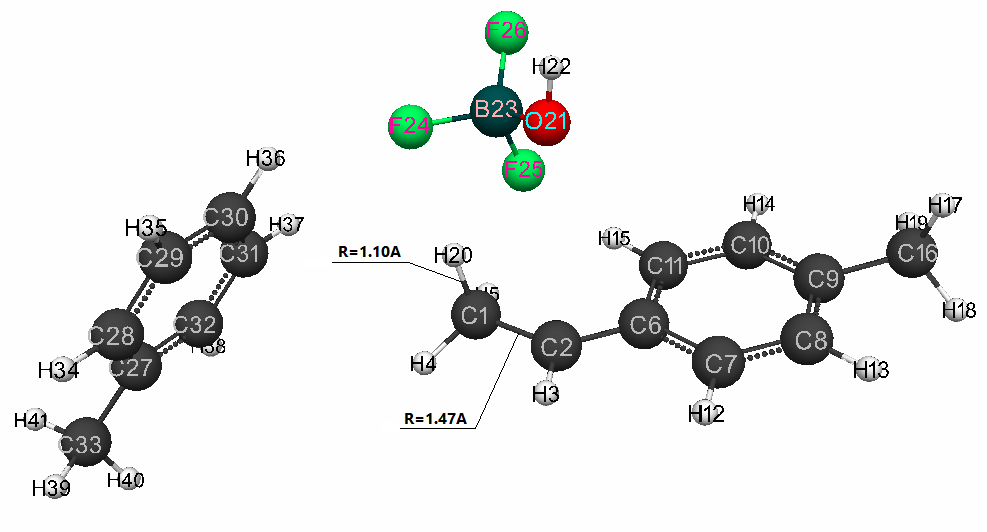
Figure 2. The final interaction structure of complex catalyst BF3·H2O in toluene (at stoichiometric composition 1:1:1) with p-methylstyrene.
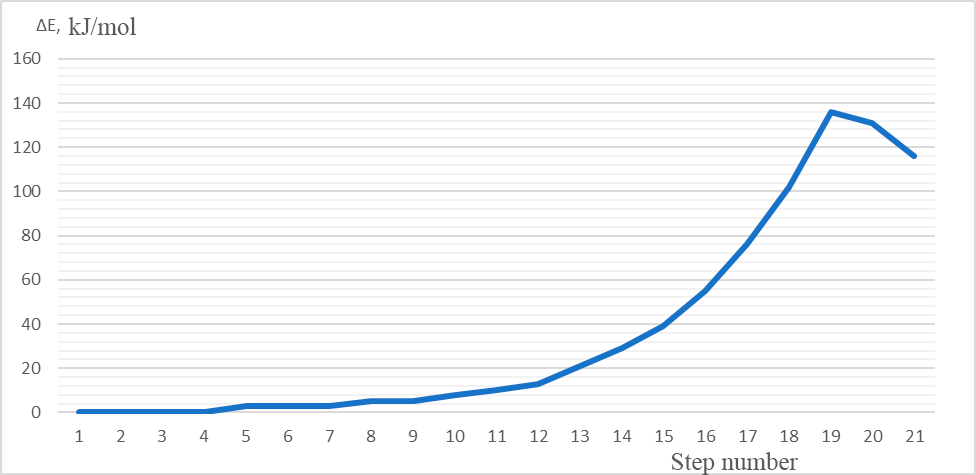
Figure 3. The total energy change (ΔE) of protonation (1-20 steps of interaction).
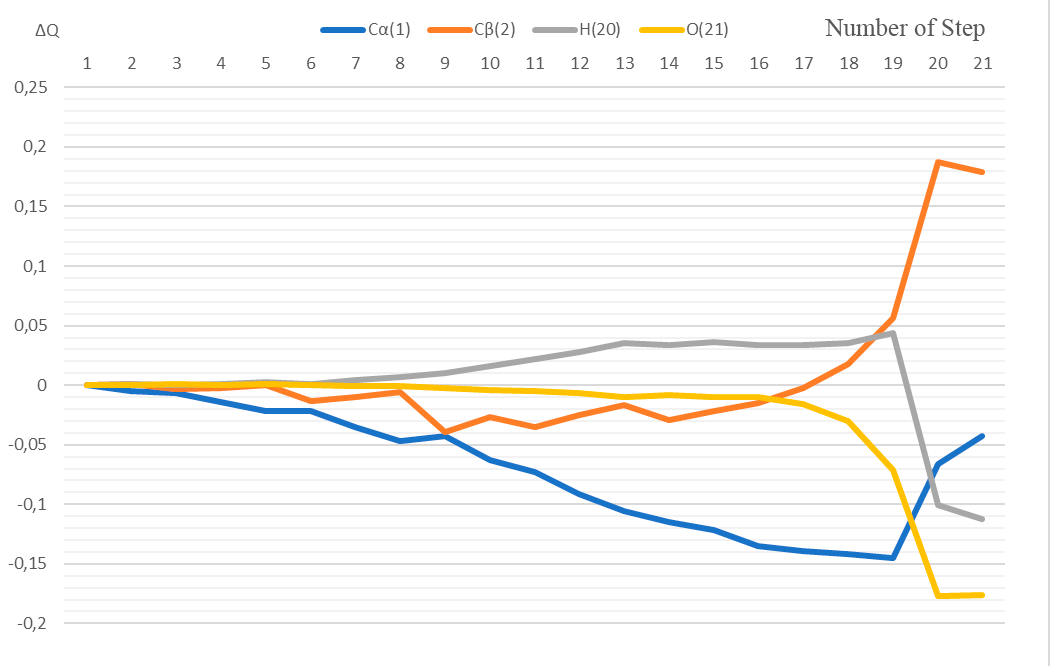
Figure 4. The change in charges along the reaction coordinate.
Table 1. The change in bond lengths as result of p-methylstyrene protonation usingcomplex catalyst BF3·H2O in toluene (stoichiometric composition 1:1:1)
|
No of step |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
C(2)-C(1) |
1,32 |
1,32 |
1,32 |
1,32 |
1,32 |
1,32 |
1,32 |
1,32 |
1,33 |
1,33 |
|
H(3)-C(2) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(4)-C(1) |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,08 |
1,08 |
1,08 |
|
H(5)-C(1) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
C(6)-C(11) |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
|
C(6)-C(2) |
1,48 |
1,48 |
1,48 |
1,48 |
1,48 |
1,48 |
1,48 |
1,48 |
1,48 |
1,48 |
|
C(7)-C(6) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
|
C(8)-C(7) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
|
C(9)-C(8) |
1,39 |
1,39 |
1,38 |
1,39 |
1,39 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
|
C(10)-C(9) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
|
C(11)-C(10) |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
|
H(12)-C(7) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(13)-C(8) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(14)-(10) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(15)-(11) |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
|
C(16)-C(9) |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
|
H(17)-(16) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(18)-(16) |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
|
H(19)-(16) |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
|
H(20)-C(1) |
3,10 |
3,00 |
2,90 |
2,80 |
2,70 |
2,60 |
2,50 |
2,40 |
2,30 |
2,20 |
|
O(21)-C(2) |
3,65 |
3,61 |
3,53 |
3,49 |
3,44 |
3,41 |
3,37 |
3,31 |
3,29 |
3,26 |
|
O(21)-(20) |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,96 |
|
H(22)-(21) |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
|
B(23)-(21) |
1,69 |
1,69 |
1,70 |
1,70 |
1,70 |
1,70 |
1,70 |
1,70 |
1,70 |
1,70 |
|
F(24)-B(23) |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
|
F(25)-B(23) |
1,35 |
1,35 |
1,35 |
1,35 |
1,35 |
1,35 |
1,35 |
1,35 |
1,35 |
1,35 |
|
F(26)-B(23) |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
|
C(27)-C(1) |
4,43 |
4,43 |
4,49 |
4,50 |
4,50 |
4,70 |
4,70 |
4,71 |
5,03 |
5,03 |
|
C(28)-C(27) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
|
C(29)-C(28) |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,39 |
1,39 |
|
C(30)-C(29) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
|
C(31)-C(30) |
1,39 |
1,38 |
1,39 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,39 |
1,39 |
|
C(32)-C(31) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
|
C(33)-C(27) |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
|
H(34)-(28) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(35)-(29) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(36)-(30) |
1,08 |
1,08 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
|
H(37)-(31) |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,08 |
1,07 |
1,08 |
1,07 |
|
H(38)-(32) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(39)-(33) |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,08 |
1,08 |
1,08 |
|
H(40)-(33) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(41)-(33) |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
|
C(2)-C(1) |
1,33 |
1,33 |
1,33 |
1,33 |
1,33 |
1,33 |
1,33 |
1,33 |
1,34 |
1,46 |
|
H(3)-C(2) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(4)-C(1) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(5)-C(1) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
C(6)-C(11) |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,42 |
Continuation of Table 1.
|
No of step |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
|
C(6)-C(2) |
1,48 |
1,48 |
1,48 |
1,48 |
1,48 |
1,48 |
1,47 |
1,47 |
1,46 |
1,38 |
1,38 |
|
C(7)-C(6) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,42 |
1,43 |
|
C(8)-C(7) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,38 |
1,36 |
1,36 |
|
C(9)-C(8) |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,39 |
1,39 |
1,41 |
1,41 |
|
C(10)-C(9) |
1,40 |
1,40 |
1,40 |
1,39 |
1,40 |
1,40 |
1,40 |
1,40 |
1,40 |
1,39 |
1,39 |
|
C(11)-C(10) |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,37 |
1,37 |
|
H(12)-C(7) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(13)-C(8) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,07 |
1,07 |
|
H(14)-C(10) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,07 |
1,07 |
|
H(15)-C(11) |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
|
C(16)-C(9) |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,50 |
1,50 |
|
H(17)-C(16) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,09 |
1,09 |
|
H(18)-C(16) |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,08 |
1,08 |
|
H(19)-C(16) |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,08 |
1,08 |
|
H(20)-C(1) |
2,10 |
2,00 |
1,90 |
1,80 |
1,70 |
1,60 |
1,50 |
1,40 |
1,30 |
1,20 |
1,10 |
|
O(21)-C(2) |
3,22 |
3,18 |
3,14 |
3,13 |
3,08 |
3,09 |
3,05 |
3,02 |
3,02 |
3,42 |
3,42 |
|
O(21)-H(20) |
0,96 |
0,96 |
0,97 |
0,98 |
0,99 |
1,00 |
1,02 |
1,06 |
1,14 |
2,46 |
2,48 |
|
H(22)-O(21) |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,95 |
0,94 |
0,94 |
0,94 |
0,94 |
|
B(23)-O(21) |
1,69 |
1,68 |
1,67 |
1,66 |
1,65 |
1,64 |
1,62 |
1,60 |
1,56 |
1,44 |
1,44 |
|
F(24)-B(23) |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,35 |
1,35 |
1,35 |
1,36 |
1,40 |
1,40 |
|
F(25)-B(23) |
1,35 |
1,35 |
1,35 |
1,35 |
1,36 |
1,36 |
1,36 |
1,37 |
1,38 |
1,42 |
1,42 |
|
F(26)-B(23) |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,34 |
1,35 |
1,35 |
1,36 |
1,38 |
1,38 |
|
C(27)-C(1) |
5,14 |
5,14 |
5,14 |
5,34 |
5,34 |
5,33 |
5,33 |
5,33 |
5,33 |
5,27 |
5,27 |
|
C(28)-C(27) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
|
C(29)-C(28) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
|
C(30)-C(29) |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
|
C(31)-C(30) |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
1,39 |
|
C(32)-C(31) |
1,39 |
1,39 |
1,38 |
1,39 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
1,38 |
|
C(33)-C(27) |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
1,51 |
|
H(34)-C(28) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(35)-C(29) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(36)-C(30) |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
|
H(37)-C(31) |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,07 |
1,08 |
1,07 |
1,07 |
|
H(38)-C(32) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(39)-C(33) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
|
H(40)-C(33) |
1,08 |
1,08 |
1,08 |
1,08 |
1,08 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
|
H(41)-C(33) |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
1,09 |
Table 2. The change in valence angles along the reaction coordinate of protonation interaction of p-methylstyrene, using complex catalyst BF3·H2O s in toluene (stoichiometric composition 1:1:1).
|
No of step |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
H(3)-C(2)-C(1) |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
|
H(4)-C(1)-C(2) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
H(5)-C(1)-C(2) |
123 |
123 |
123 |
123 |
123 |
123 |
123 |
123 |
123 |
123 |
|
C(6)-C(11)-C(10) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
C(6)-C(2)-C(1) |
127 |
127 |
127 |
127 |
127 |
127 |
127 |
127 |
128 |
128 |
|
C(7)-C(6)-C(11) |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
|
C(8)-C(7)-C(6) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
C(9)-C(8)-C(7) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
C(10)-C(9)-C(8) |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
|
C(11)-C(10)-C(9) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
H(12)-C(7)-C(6) |
119 |
119 |
119 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
H(13)-C(8)-C(7) |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
|
H(14)-C(10)-C(9) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
H(15)-C(11)-C(10) |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
|
C(16)-C(9)-C(8) |
122 |
122 |
122 |
122 |
122 |
122 |
122 |
122 |
122 |
122 |
|
H(17)-C(16)-C(9) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
|
H(18)-C(16)-C(9) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
|
H(19)-C(16)-C(9) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
|
H(20)-C(1)-C(2) |
88 |
88 |
87 |
87 |
87 |
84 |
85 |
85 |
82 |
84 |
|
O(21)-C(2)-C(1) |
77 |
76 |
77 |
76 |
76 |
76 |
75 |
74 |
74 |
72 |
|
H(22)-O(21)-C(2) |
48 |
51 |
53 |
56 |
58 |
65 |
67 |
69 |
79 |
81 |
|
B(23)-O(21)-C(2) |
121 |
121 |
123 |
123 |
124 |
125 |
125 |
126 |
125 |
126 |
|
F(24)-B(23)-O(21) |
102 |
102 |
102 |
103 |
103 |
103 |
103 |
103 |
103 |
103 |
|
F(25)-B(23)-O(21) |
103 |
103 |
103 |
103 |
103 |
103 |
103 |
103 |
103 |
103 |
|
F(26)-B(23)-O(21) |
101 |
101 |
101 |
101 |
101 |
101 |
101 |
101 |
101 |
101 |
|
C(28)-C(27)-C(1) |
98 |
98 |
96 |
96 |
96 |
95 |
94 |
94 |
91 |
91 |
|
C(29)-C(28)-C(27) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
C(30)-C(29)-C(28) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
C(31)-C(30)-C(29) |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
|
C(32)-C(31)-C(30) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
C(33)-C(27)-C(1) |
100 |
100 |
104 |
104 |
104 |
110 |
110 |
110 |
118 |
118 |
|
H(34)-C(28)-C(27) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
H(35)-C(29)-C(28) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
H(36)-C(30)-C(29) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
H(37)-C(31)-C(30) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
H(38)-C(32)-C(31) |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
|
H(39)-C(33)-C(27) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
|
H(40)-C(33)-C(27) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
|
H(41)-C(33)-C(27) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
|
H(3)-C(2)-C(1) |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
117 |
115 |
|
H(4)-C(1)-C(2) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
119 |
112 |
|
H(5)-C(1)-C(2) |
123 |
123 |
123 |
123 |
123 |
123 |
122 |
122 |
122 |
115 |
|
C(6)-C(11)-C(10) |
121 |
121 |
121 |
121 |
121 |
121 |
120 |
120 |
120 |
120 |
|
C(6)-C(2)-C(1) |
128 |
128 |
128 |
128 |
128 |
128 |
128 |
128 |
128 |
129 |
|
C(7)-C(6)-C(11) |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
119 |
119 |
|
C(8)-C(7)-C(6) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
C(9)-C(8)-C(7) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
120 |
|
C(10)-C(9)-C(8) |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
118 |
120 |
Continuation of Table 2.
|
No of step |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
|
C(11)-C(10)-C(9) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
H(12)-C(7)-C(6) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
119 |
119 |
|
H(13)-C(8)-C(7) |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
120 |
121 |
121 |
|
H(14)-C(10)-C(9) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
H(15)-C(11)-C(10) |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
120 |
120 |
|
C(16)-C(9)-C(8) |
122 |
122 |
122 |
122 |
122 |
122 |
122 |
122 |
121 |
119 |
119 |
|
H(17)-C(16)-C(9) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
109 |
109 |
|
H(18)-C(16)-C(9) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
|
H(19)-C(16)-C(9) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
112 |
112 |
|
H(20)-C(1)-C(2) |
84 |
86 |
87 |
89 |
91 |
94 |
96 |
97 |
96 |
103 |
105 |
|
O(21)-C(2)-C(1) |
70 |
68 |
65 |
61 |
60 |
56 |
54 |
52 |
52 |
67 |
66 |
|
H(22)-O(21)-C(2) |
86 |
87 |
87 |
92 |
92 |
92 |
92 |
93 |
94 |
138 |
137 |
|
B(23)-O(21)-C(2) |
126 |
127 |
128 |
127 |
128 |
127 |
128 |
128 |
126 |
110 |
110 |
|
F(24)-B(23)-O(21) |
103 |
103 |
104 |
103 |
104 |
103 |
104 |
105 |
105 |
111 |
111 |
|
F(25)-B(23)-O(21) |
103 |
103 |
104 |
104 |
104 |
104 |
105 |
105 |
106 |
107 |
107 |
|
F(26)-B(23)-O(21) |
101 |
101 |
101 |
102 |
102 |
103 |
103 |
104 |
105 |
112 |
112 |
|
C(28)-C(27)-C(1) |
89 |
89 |
89 |
90 |
90 |
87 |
87 |
88 |
88 |
87 |
87 |
|
C(29)-C(28)-C(27) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
C(30)-C(29)-C(28) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
C(31)-C(30)-C(29) |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
|
C(32)-C(31)-C(30) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
C(33)-C(27)-C(1) |
121 |
121 |
121 |
125 |
125 |
128 |
128 |
128 |
128 |
132 |
132 |
|
H(34)-C(28)-C(27) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
H(35)-C(29)-C(28) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
|
H(36)-C(30)-C(29) |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
121 |
|
H(37)-C(31)-C(30) |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
120 |
119 |
119 |
|
H(38)-C(32)-C(31) |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
119 |
|
H(39)-C(33)-C(27) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
|
H(40)-C(33)-C(27) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
|
H(41)-C(33)-C(27) |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
111 |
Table 3. The change in charges along the reaction interaction path of complex catalyst BF3·H2O in toluene (stoichiometric composition 1:1:1) with p-methylstyrene.
|
Atom |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
C(1) |
-0,219 |
-0,224 |
-0,226 |
-0,233 |
-0,241 |
-0,241 |
-0,254 |
-0,266 |
-0,262 |
-0,282 |
|
C(2) |
-0,098 |
-0,097 |
-0,101 |
-0,100 |
-0,098 |
-0,111 |
-0,108 |
-0,104 |
-0,137 |
-0,125 |
|
H(3) |
0,100 |
0,100 |
0,101 |
0,102 |
0,103 |
0,102 |
0,103 |
0,104 |
0,105 |
0,106 |
|
H(4) |
0,120 |
0,121 |
0,123 |
0,125 |
0,126 |
0,132 |
0,133 |
0,135 |
0,144 |
0,145 |
|
H(5) |
0,135 |
0,136 |
0,136 |
0,139 |
0,141 |
0,140 |
0,144 |
0,148 |
0,146 |
0,152 |
|
C(6) |
-0,130 |
-0,132 |
-0,139 |
-0,140 |
-0,143 |
-0,127 |
-0,130 |
-0,134 |
-0,103 |
-0,109 |
|
C(7) |
-0,060 |
-0,060 |
-0,060 |
-0,059 |
-0,059 |
-0,054 |
-0,054 |
-0,054 |
-0,048 |
-0,048 |
|
C(8) |
-0,077 |
-0,077 |
-0,076 |
-0,076 |
-0,076 |
-0,078 |
-0,079 |
-0,079 |
-0,082 |
-0,082 |
|
C(9) |
-0,124 |
-0,124 |
-0,126 |
-0,126 |
-0,126 |
-0,125 |
-0,125 |
-0,125 |
-0,122 |
-0,122 |
|
C(10) |
-0,060 |
-0,060 |
-0,062 |
-0,062 |
-0,062 |
-0,070 |
-0,070 |
-0,070 |
-0,080 |
-0,080 |
|
C(11) |
-0,143 |
-0,140 |
-0,123 |
-0,119 |
-0,114 |
-0,112 |
-0,104 |
-0,100 |
-0,104 |
-0,096 |
|
H(12) |
0,093 |
0,093 |
0,093 |
0,093 |
0,094 |
0,094 |
0,094 |
0,094 |
0,094 |
0,094 |
|
H(13) |
0,092 |
0,092 |
0,092 |
0,092 |
0,092 |
0,092 |
0,092 |
0,092 |
0,092 |
0,092 |
|
H(14) |
0,101 |
0,101 |
0,100 |
0,100 |
0,100 |
0,100 |
0,100 |
0,100 |
0,098 |
0,098 |
|
H(15) |
0,119 |
0,119 |
0,116 |
0,116 |
0,116 |
0,117 |
0,117 |
0,117 |
0,124 |
0,123 |
|
C(16) |
-0,175 |
-0,175 |
-0,175 |
-0,175 |
-0,176 |
-0,176 |
-0,176 |
-0,176 |
-0,176 |
-0,176 |
|
H(17) |
0,095 |
0,095 |
0,095 |
0,095 |
0,096 |
0,096 |
0,096 |
0,096 |
0,097 |
0,097 |
|
H(18) |
0,112 |
0,112 |
0,112 |
0,112 |
0,112 |
0,113 |
0,113 |
0,114 |
0,115 |
0,115 |
|
H(19) |
0,116 |
0,116 |
0,116 |
0,116 |
0,116 |
0,113 |
0,114 |
0,114 |
0,110 |
0,111 |
|
H(20) |
0,325 |
0,326 |
0,325 |
0,326 |
0,328 |
0,326 |
0,329 |
0,332 |
0,335 |
0,341 |
|
O(21) |
-0,437 |
-0,437 |
-0,436 |
-0,437 |
-0,436 |
-0,437 |
-0,438 |
-0,438 |
-0,439 |
-0,441 |
|
H(22) |
0,336 |
0,334 |
0,332 |
0,331 |
0,329 |
0,324 |
0,323 |
0,322 |
0,315 |
0,314 |
|
B(23) |
0,820 |
0,822 |
0,819 |
0,821 |
0,822 |
0,817 |
0,818 |
0,819 |
0,810 |
0,810 |
|
F(24) |
-0,340 |
-0,339 |
-0,339 |
-0,338 |
-0,337 |
-0,335 |
-0,334 |
-0,334 |
-0,332 |
-0,333 |
|
F(25) |
-0,360 |
-0,360 |
-0,359 |
-0,359 |
-0,360 |
-0,356 |
-0,357 |
-0,358 |
-0,354 |
-0,356 |
|
F(26) |
-0,340 |
-0,341 |
-0,340 |
-0,341 |
-0,343 |
-0,339 |
-0,342 |
-0,344 |
-0,339 |
-0,342 |
|
C(27) |
-0,123 |
-0,124 |
-0,124 |
-0,124 |
-0,125 |
-0,123 |
-0,124 |
-0,124 |
-0,121 |
-0,121 |
|
C(28) |
-0,092 |
-0,093 |
-0,094 |
-0,094 |
-0,094 |
-0,097 |
-0,097 |
-0,097 |
-0,098 |
-0,098 |
|
C(29) |
-0,077 |
-0,080 |
-0,078 |
-0,081 |
-0,083 |
-0,081 |
-0,082 |
-0,084 |
-0,085 |
-0,086 |
|
C(30) |
-0,176 |
-0,166 |
-0,169 |
-0,158 |
-0,149 |
-0,148 |
-0,141 |
-0,135 |
-0,131 |
-0,128 |
|
C(31) |
-0,112 |
-0,116 |
-0,110 |
-0,112 |
-0,116 |
-0,107 |
-0,109 |
-0,111 |
-0,102 |
-0,103 |
|
C(32) |
-0,084 |
-0,084 |
-0,087 |
-0,088 |
-0,089 |
-0,095 |
-0,095 |
-0,096 |
-0,102 |
-0,102 |
|
C(33) |
-0,178 |
-0,178 |
-0,177 |
-0,177 |
-0,177 |
-0,176 |
-0,176 |
-0,176 |
-0,176 |
-0,176 |
|
H(34) |
0,089 |
0,088 |
0,088 |
0,088 |
0,087 |
0,086 |
0,086 |
0,086 |
0,085 |
0,085 |
|
H(35) |
0,100 |
0,099 |
0,099 |
0,098 |
0,098 |
0,096 |
0,096 |
0,096 |
0,094 |
0,094 |
|
H(36) |
0,122 |
0,120 |
0,124 |
0,122 |
0,121 |
0,121 |
0,120 |
0,118 |
0,116 |
0,115 |
|
H(37) |
0,129 |
0,129 |
0,126 |
0,125 |
0,125 |
0,124 |
0,123 |
0,123 |
0,121 |
0,121 |
|
H(38) |
0,092 |
0,092 |
0,091 |
0,091 |
0,090 |
0,089 |
0,089 |
0,088 |
0,087 |
0,087 |
|
H(39) |
0,100 |
0,100 |
0,099 |
0,099 |
0,098 |
0,098 |
0,098 |
0,097 |
0,096 |
0,096 |
|
H(40) |
0,096 |
0,096 |
0,096 |
0,096 |
0,096 |
0,096 |
0,096 |
0,095 |
0,096 |
0,096 |
|
H(41) |
0,115 |
0,115 |
0,115 |
0,115 |
0,114 |
0,114 |
0,114 |
0,114 |
0,113 |
0,113 |
|
C(1) |
-0,292 |
-0,310 |
-0,325 |
-0,334 |
-0,341 |
-0,354 |
-0,358 |
-0,361 |
-0,364 |
-0,285 |
|
C(2) |
-0,133 |
-0,123 |
-0,115 |
-0,127 |
-0,120 |
-0,113 |
-0,100 |
-0,080 |
-0,042 |
0,089 |
|
H(3) |
0,108 |
0,109 |
0,110 |
0,112 |
0,114 |
0,115 |
0,117 |
0,121 |
0,128 |
0,144 |
|
H(4) |
0,152 |
0,154 |
0,157 |
0,166 |
0,169 |
0,178 |
0,180 |
0,182 |
0,181 |
0,140 |
|
H(5) |
0,153 |
0,158 |
0,163 |
0,164 |
0,168 |
0,171 |
0,175 |
0,181 |
0,187 |
0,175 |
|
C(6) |
-0,102 |
-0,106 |
-0,110 |
-0,087 |
-0,094 |
-0,091 |
-0,100 |
-0,112 |
-0,131 |
-0,204 |
Continuation of Table 3.
|
Atom |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
|
C(7) |
-0,047 |
-0,046 |
-0,044 |
-0,044 |
-0,042 |
-0,042 |
-0,038 |
-0,034 |
-0,025 |
0,032 |
0,034 |
|
C(8) |
-0,083 |
-0,083 |
-0,084 |
-0,085 |
-0,085 |
-0,086 |
-0,087 |
-0,089 |
-0,092 |
-0,127 |
-0,128 |
|
C(9) |
-0,122 |
-0,121 |
-0,120 |
-0,118 |
-0,117 |
-0,115 |
-0,113 |
-0,110 |
-0,102 |
-0,039 |
-0,036 |
|
C(10) |
-0,084 |
-0,084 |
-0,084 |
-0,089 |
-0,089 |
-0,091 |
-0,092 |
-0,094 |
-0,099 |
-0,137 |
-0,138 |
|
C(11) |
-0,087 |
-0,084 |
-0,080 |
-0,083 |
-0,078 |
-0,069 |
-0,064 |
-0,056 |
-0,042 |
0,114 |
0,118 |
|
H(12) |
0,094 |
0,094 |
0,094 |
0,094 |
0,094 |
0,094 |
0,095 |
0,097 |
0,100 |
0,117 |
0,118 |
|
H(13) |
0,091 |
0,092 |
0,092 |
0,092 |
0,092 |
0,092 |
0,093 |
0,094 |
0,097 |
0,110 |
0,110 |
|
H(14) |
0,098 |
0,098 |
0,098 |
0,097 |
0,098 |
0,098 |
0,099 |
0,101 |
0,104 |
0,123 |
0,124 |
|
H(15) |
0,124 |
0,124 |
0,124 |
0,129 |
0,130 |
0,130 |
0,133 |
0,138 |
0,151 |
0,216 |
0,218 |
|
C(16) |
-0,176 |
-0,176 |
-0,177 |
-0,177 |
-0,177 |
-0,177 |
-0,178 |
-0,178 |
-0,179 |
-0,188 |
-0,188 |
|
H(17) |
0,096 |
0,097 |
0,097 |
0,097 |
0,097 |
0,097 |
0,098 |
0,098 |
0,100 |
0,140 |
0,140 |
|
H(18) |
0,114 |
0,114 |
0,114 |
0,115 |
0,115 |
0,114 |
0,115 |
0,116 |
0,118 |
0,122 |
0,123 |
|
H(19) |
0,112 |
0,112 |
0,112 |
0,111 |
0,112 |
0,113 |
0,114 |
0,116 |
0,119 |
0,122 |
0,123 |
|
H(20) |
0,347 |
0,353 |
0,360 |
0,359 |
0,361 |
0,359 |
0,359 |
0,360 |
0,369 |
0,224 |
0,213 |
|
O(21) |
-0,442 |
-0,444 |
-0,447 |
-0,445 |
-0,447 |
-0,447 |
-0,453 |
-0,467 |
-0,508 |
-0,614 |
-0,613 |
|
H(22) |
0,311 |
0,310 |
0,309 |
0,306 |
0,305 |
0,302 |
0,300 |
0,296 |
0,290 |
0,244 |
0,243 |
|
B(23) |
0,805 |
0,805 |
0,804 |
0,800 |
0,798 |
0,797 |
0,794 |
0,790 |
0,787 |
0,810 |
0,809 |
|
F(24) |
-0,334 |
-0,335 |
-0,337 |
-0,342 |
-0,344 |
-0,350 |
-0,355 |
-0,361 |
-0,373 |
-0,440 |
-0,439 |
|
F(25) |
-0,356 |
-0,358 |
-0,360 |
-0,362 |
-0,366 |
-0,369 |
-0,375 |
-0,383 |
-0,399 |
-0,466 |
-0,467 |
|
F(26) |
-0,341 |
-0,343 |
-0,346 |
-0,344 |
-0,347 |
-0,347 |
-0,352 |
-0,358 |
-0,368 |
-0,408 |
-0,408 |
|
C(27) |
-0,120 |
-0,120 |
-0,120 |
-0,120 |
-0,120 |
-0,120 |
-0,120 |
-0,120 |
-0,120 |
-0,122 |
-0,122 |
|
C(28) |
-0,097 |
-0,098 |
-0,098 |
-0,097 |
-0,097 |
-0,095 |
-0,096 |
-0,096 |
-0,097 |
-0,099 |
-0,099 |
|
C(29) |
-0,087 |
-0,087 |
-0,088 |
-0,087 |
-0,087 |
-0,087 |
-0,088 |
-0,089 |
-0,090 |
-0,088 |
-0,088 |
|
C(30) |
-0,124 |
-0,122 |
-0,120 |
-0,121 |
-0,119 |
-0,122 |
-0,120 |
-0,119 |
-0,118 |
-0,131 |
-0,130 |
|
C(31) |
-0,101 |
-0,102 |
-0,103 |
-0,107 |
-0,107 |
-0,110 |
-0,110 |
-0,110 |
-0,111 |
-0,104 |
-0,105 |
|
C(32) |
-0,104 |
-0,104 |
-0,104 |
-0,102 |
-0,102 |
-0,101 |
-0,101 |
-0,102 |
-0,104 |
-0,106 |
-0,106 |
|
C(33) |
-0,176 |
-0,176 |
-0,176 |
-0,177 |
-0,177 |
-0,177 |
-0,176 |
-0,176 |
-0,176 |
-0,176 |
-0,176 |
|
H(34) |
0,084 |
0,084 |
0,084 |
0,084 |
0,084 |
0,084 |
0,084 |
0,084 |
0,083 |
0,079 |
0,079 |
|
H(35) |
0,093 |
0,093 |
0,092 |
0,092 |
0,092 |
0,092 |
0,092 |
0,092 |
0,091 |
0,089 |
0,089 |
|
H(36) |
0,113 |
0,113 |
0,112 |
0,109 |
0,109 |
0,110 |
0,110 |
0,111 |
0,112 |
0,126 |
0,126 |
|
H(37) |
0,120 |
0,121 |
0,121 |
0,124 |
0,125 |
0,126 |
0,126 |
0,128 |
0,131 |
0,139 |
0,139 |
|
H(38) |
0,087 |
0,087 |
0,087 |
0,087 |
0,087 |
0,087 |
0,087 |
0,087 |
0,087 |
0,083 |
0,083 |
|
H(39) |
0,095 |
0,095 |
0,095 |
0,095 |
0,095 |
0,093 |
0,093 |
0,093 |
0,092 |
0,090 |
0,090 |
|
H(40) |
0,097 |
0,097 |
0,097 |
0,097 |
0,097 |
0,099 |
0,099 |
0,099 |
0,098 |
0,094 |
0,094 |
|
H(41) |
0,113 |
0,113 |
0,112 |
0,112 |
0,112 |
0,112 |
0,112 |
0,112 |
0,112 |
0,111 |
0,110 |
References
- Kennedy, J., Cationic polymerization of olefins. “Mir” Publishing House, Moscow, 1978, 431 p. (in Russian).
- Cirelson V.G., Quantum Chemistry. Molecules, molecular systems and solids, Moscow, Publishing House «Binom», 2010, 496 p. (in Russian).
- Granovsky, A.A., Firefly version 8, 2013. http://classic.chem.msu.su/gran/firefly/index.html
- General Atomic and Molecular Electronic Structure System., M.W. Schmidt [and others], J.Comput.Chem., 1993, 14, 1347-1363.
- Potential Energy Surface of Interaction between Ethriolbicyclophosphite and Acetyl Chloride (Second Stage), V. A. Babkin [and others], Oxidation Communications. 2018, 41(2), 231-239.
- On the Mechanism of Cationic Polymerisation of P-Isopropylstyrene in the Presence of a Complex Catalyst Boron Fluoride-Water, V. A. Babkin [and others], Oxidation Communications, 2019, 42(1), 56‑62.
- Quantum-chemical study of the mechanism of protonation 2,3,4,5-tetramethylstyrene by method AB INITIO, V. A. Babkin [and others], Izvestia Vstu,Volgograd, 2019, 5. 22-28 (Series: "Chemistry and Technology of Organoelement Monomers and Polymer Materials "; vol. 228, (in Russian)).
- About the initiation mechanism of cationic polymerization of p-ethylstyrene in the presence of a complex catalyst boron fluorine – water., V. A. Babkin [and others], Fluorine Notes, 2019, 3(124), 3–4.
- Quantum chemical investigation of the initiation mechanism of the cationic polymerisation of 4-methylpentene-1 with chloride–aluminum aquacomplex / V. A. Babkin [and others] // Oxidation Communications. - 2019, -Vol. 42, No 3, PP. 275–281.
- MacMolPlt: A Graphical User Interface for GAMESS., B.M. Bode, M.S. Gordon, Journal of Molecular Graphics, 1998, 16, 133-138.
ARTICLE INFO
Received 09 December 2019
Accepted 12 February 2020
Available online February 2020
Recommended for publication by PhD Marina A. Manaenkova
Fluorine Notes, 2020, 129, 3-4
