Received: April 2020
DOI 10.17677/fn20714807.2020.03.01
Fluorine Notes, 2020, 130, 1-2
THREE SERIES IONS OF PERFLUOROTRIBUTYLAMINE MASS-SPECTRUM (PFTBA)
N.D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: The spectral analysis of perfluorotributylamine identified three main series of ions, that are generated during the fragmentation of PFTBA molecular cation radicals, as well as the three subseries formed as a result of additional, symmetrical, stabilizing detachments of fluorine atoms from the ions [C4F9]+ and [N(C4F8)3]+ of the principal spectral series. For each of the series, the sequence of .F and groups of CF2 detachments correspond to the sequence of mass numbers of generated ions. Using the PFTBA spectrum as an example, the validity and flexibility of earlier proposed algorithm is confirmed for the fragmentation of n-perfluoroalkanes.
Keywords: fragmentation algorithm, mass spectrum of perfluorotributylamine, PFTBA, n-perfluoroalkanes, molecular radical cation, series of ions.
Introduction
Perfluorotributylamine (PFTBA) is the standard for the tuning of mass spectrometers with a mass range of up to 1000 amu based on reference peaks [1,2]. Perhaps, for this reason its mass-spectrum (NIST #: 66003 ID #: 8854 DB: replib Contributer: G.W.A. MILNE, NAT’L INSTITUTES OF HEALTH, USA) is the most high-quality and detailed, of all the spectra of tertiary perfluoramines presented in the NIST libraries.
In contrast to the publications on spectra of amines and tertiary amines [3,4], the detailed analysis of the unique PFTBA spectrum, apparently was not performed. Thus, it was of the utmost interest, to verify the applicability of fragmentation algorithm proposed earlier for n-perfluoroalkanes [5], using PFTBA as an example.
In the spectra of n-perfluoroalkanes, the alkyl [CnF2n+1]+ series appears as a result of initial detachment of fluorine atom from CF3 group and subsequent emissions of CF2. A less intense alkenyl [CnF2n-2]+ series begins with the three consecutive detachments of a fluorine atom (M-57). The emission of first fluorine atom occurs as in alkyl series, with the addition of the two symmetrical detachments of a fluorine atom from the opposite terminal group C2F5 with the formation of a stabilizing vinyl group.
In comparison to the spectra of n-perfluoroalkanes, PFTBA spectrum with a central nitrogen atom, fixing the end groups of CF2, is interesting for the occurring changes in fragmentation. PFTBA spectrum analysis, conducted using the n-perfluoroalkane algorithm [5], made it possible to isolate the three main series of ions within its spectrum, in addition to three additional subseries formed as a result of symmetric detachments of fluorine atoms from the terminal group C2F5 of the [C4F9]+ ion, as well as from the C2F4 groups of the [N(C4F8)3]+ ion adjacent to the nitrogen atom, and to confirm the sequences of the decay paths and the applicability of the algorithm, proposed previously for fragmentation of n-perfluoroalkanes.
Fragmentation of PFTBA
The two main series of ions, achieve maximum peak intensities in the total ion current of PFTBA spectrum are formed during the detachments of (C4F9)2 :N .. α-CF2 and (C4F9)2 : N-CF2 .. β-CF2 σ-bonds of the central group of PFTBA atoms.
M – e → C4F9+ . N(C4F9)2 → C4F9+ + .N(C4F9)2 m=452
M – e → C3F7 . +CF2N(C4F9)2 → +CF2N(C4F9)2 + .C3F7 m=169
As judged by the values of the total ion current of the spectrum, the probabilities of formation of these two series are correlated as 4:1.
N(C4F9)3 MW=671 (series M- . N(C4F9)2) N1
detachmnet of nitrogen radical with two substituents
(strongest alkyl-olefin series)
|
671 |
M-.N(C4F9)2 |
=219 |
[C4F9]+ |
66,6% |
|
219 |
- CF2 |
=169 |
[C3F7]+ |
3,9% |
|
*169 |
- F |
=150 |
[C3F6]+ |
2,4% |
|
219 |
- CF2 |
=119 |
[C2F5]+ |
11,0% |
|
*119 |
- F |
=100 |
[C2F4]+ |
13,5% |
|
119 |
- CF2 |
=69 |
[CF3]+ |
100 % |
|
Ion current series: 197,4 % (46.90% of total ion current of the spectrum) |
||||
As a result of two stabilizing detachments .F, two olefin ions: 169 * [C3F6 ]+ and 119 * [C2F4]+, are formed within the N1 series, that consists out of four alkyl ions.
The series of perfluoroalkyl ions N1 are accompanied by the alkenyl subseries N1*, the probability of which is 1.8 less than the probability of the main series.
The alkenyl subseries N1* differs from the main series N1 by the additional, symmetrical and stabilizing detachment of two fluorine atoms from terminal C2F5 group, with the formation of vinyl group [CF2= CF2-CF2-CF2]+. The resulting [C4F9]+ ion, the N1 series of fragmented alkyl ions, and alkenyl subseries N1* completely repeat the fragmentation of linear perfluoroalkanes [5].
N(C4F9)3 MW=671 (subseries M- . N(C4F9)2 -2.F ) N1*
detachment of nitrogen radical with two substituents and two fluorine atoms
(the alkyl series N1 and the alkenyl subseries N1 * have a common ion with m/z 219)
|
671 |
M -.N(C4F9)2 |
=219 |
[CF3-CF2-CF2-CF2]+ |
219 |
66,6% |
|
|
219 |
- 2.F |
=181 |
[CF2=CF-CF2-CF2]+ |
181 |
3,1% |
|
|
181 |
- CF2 |
=131 |
[CF2=CF-CF2]+ |
131 |
41,6% |
|
|
131 |
- CF2 |
=81 |
[CF2=CF]+ |
81 |
0,4% |
|
|
Ion current series 111,7 % (26,53% of total ion current in the spectrum |
||||||
The second main (third in intensity) series of ion peaks is formed during the separation of +.[M] – . C3F7 = [CF2N(C4F9)2]+ 502 11,4 % radical, and subsequent detachments of two fluorine atoms from two terminal groups of CF3 perfluoroalkyl substituents, and seven emissions of CF2 groups.
N(C4F9)3 MW=671 (series M- . C3F7 -2.F) N2
detachment of perfluoroalkyl radical C4F9 without one CF2 group, two fluorine atoms from two groups of CF3 and the carbene "leaf fall" of seven CF2 groups
(alkyl nitrogen-containing series N2, and series N3 M-3F have a common ion with m/z 464)
|
671 |
M- C3F7 |
=502 |
[CF2-N-(C4F9)2]+ |
11,4% |
|
502 |
-2.F |
=464 |
[CF2-N-(C4F8)2 ]+ |
6,4% |
|
*464 |
-CF2 |
=414 |
[CF2-N(C3F6)(C4F8)]+ |
7,0% |
|
414 |
-CF2 |
=364 |
[CF2-N-(C3F6)2]+ |
0,6% |
|
364 |
-CF2 |
=314 |
[CF2-N-(C2F4)(C3F6)]+ |
1,0% |
|
314 |
-CF2 |
=264 |
[CF2-N-(C2F4)2]+ |
15,2% |
|
264 |
-CF2 |
=214 |
[(CF2)2N-C2F4]+ |
1,2% |
|
214 |
-CF2 |
=164 |
[(CF2)3N]+ |
1,2 % |
|
164 |
-CF2 |
=114 |
[CF2-N-CF2]+ |
4,1 % |
|
Ion current series 48,1 % (11,42% of total ion current in the spectrum) |
||||
The least intense main series of PFTBA spectrum is the N3 series, which occurs when three fluorine atoms are detached from three terminal CF3 groups of three substituents. The detachment of one fluorine atom from one of CF3 groups, apparently, initiates the symmetrical detachments of two fluorine atoms from two other CF3 groups. The probability of the formation of this series is 4.7 times less than the probability of the main series N1.
N(C4F9)3 MW=671 (series M- 3.F) N3
nitrogen-containing alkyl series
(separation of three fluorine atoms from three end groups of CF3 of three substituents and the carbene "leaf fall" of ten CF2 groups)
|
671 |
(M-3.F) |
=614 |
[N(C4F8)3]+ |
4,1% |
|
614 |
- CF2 |
=564 |
[(C3F6)-N(C4F8)2]+ |
0,4% |
|
564 |
- CF2 |
=514 |
[(C3F6)2N-C4F8]+ |
0,4% |
|
514 |
- CF2 |
=464 |
[N(C3F6)3]+ |
6,4% |
|
*464 |
- CF2 |
=414 |
[C2F4-N(C3F6)2]+ |
7,0% |
|
414 |
- CF2 |
=364 |
[(C2F4)2N-C3F6]+ |
0,6% |
|
364 |
- CF2 |
=314 |
[N(C2F4)3]+ |
1,0% |
|
314 |
- CF2 |
=264 |
[(C2F4)2N-CF2]+ |
15,2% |
|
264 |
- CF2 |
=214 |
[(CF2)2N-C2F4]+ |
1,2% |
|
214 |
- CF2 |
=164 |
[(CF2)3N]+ |
1,2% |
|
164 |
- CF2 |
=114 |
[CF2-N-CF2]+ |
4,1% |
|
Ion current series 41,6 % (9,89% of total ion current in the spectrum) |
||||
Series N3 +.[M] –3 .F 4.1% is accompanied by two concomitant alkenyl subseries N3* and N3**, formed as a result of additional stabilizing detachments of two fluorine atoms from group CF2-CF2-N of an adjacent nitrogen atom, one of substituents and, accordingly, the detachment of four fluorine atoms from two groups CF2-CF2-N of two substituents of [N(C4F8)3]+ ion, adjacent to the nitrogen.
The intensities and probabilities of these two alkenyl subseries are 2.9 and 5.2 times less than the intensities of the series +.[M] –3 .F.
The detachment of first fluorine atom from the α-CF2N substituent group, probably initiates the stabilizing detachment of second fluorine atom from the β- CF2 group.
N(C4F9)3 MW=671 (series M- 5.F) N3*
detachment of three fluorine atoms from three terminal groups of CF3, and then - another two fluorine atoms from adjacent CF2CF2 group of one of a substituents
(nitrogen-containing series N3 and N3* have a common ion with m/z 614)
|
671 |
(M-3.F) |
=614 |
[N(C4F8)3]+ |
4,1% |
|
614 |
-2.F |
=576 |
[CF2-CF2-CF=CF-N(C4F8)2]+ |
2,0% |
|
576 |
-CF2 |
=526 |
[CF2 -CF=CF-N(C4F8)2]+ |
0,4% |
|
526 |
-CF2 |
=476 |
[CF=CF-N(C4F8)2]+ |
0,4% |
|
476 |
-CF2 |
=426 |
[CF=CF-N(C3F6)(C4F8)]+ |
2,4% |
|
426 |
-CF2 |
=376 |
[CF=CF-N(C3F6)2]+ |
1,4% |
|
376 |
-CF2 |
=326 |
[CF=CF-N(C2F4)(C3F6)]+ |
0,6% |
|
326 |
-CF2 |
=276 |
[CF=CF-N(C2F4)2]+ |
0,4% |
|
276 |
-CF2 |
=226 |
[CF=CF-N(CF2)(C2F4)]+ |
0,6% |
|
226 |
-CF2 |
=176 |
[CF=CF-N(CF2)2]+ |
1,2% |
|
176 |
-CF2 |
=126 |
[CF=CF-N-CF2]+ |
0,4% |
|
126 |
-CF2 |
=76 |
[CF=N=CF]+ |
0,4% |
|
Ion current series 4,7 % (3,39% of total ion current in the spectrum) |
||||
N(C4F9)3 MW=671 (series M-7.F ) N3**
detachment of three fluorine atoms from three terminal CF3 groups of three substituents and two fluorine atoms from each of two CF2CF2 groups adjacent to the nitrogen atom of two substituents
(nitrogen-containing series N3, N3* and N3** have a common ion with m/z 614)
|
671 |
(M-3F) |
=614 |
[N(C4F8)3]+ |
4,1% |
|
614 |
-2F |
=576 |
[(CF2-CF2-CF=CF)N(C4F8)2]+ |
0,6% |
|
576 |
-2F |
=538 |
[(CF2-CF2-CF=CF)2N(C4F8)]+ |
0,6% |
|
538 |
-CF2 |
=488 |
[CF2-CF=CF-N(C4F8)(CF=CF-CF2-CF2)]+ |
0,4% |
|
488 |
-CF2 |
=438 |
[(CF2-CF=CF)2N(C4F8)]+ |
0,2% |
|
438 |
-CF2 |
=388 |
[CF=CF-N(C4F8)(CF=CF-CF2)]+ |
0,4% |
|
388 |
-CF2 |
=338 |
[(CF=CF)2N(C4F8)]+ |
0,4% |
|
338 |
-CF2 |
=288 |
[(CF=CF)2N(C3F6)]+ |
0,2% |
|
288 |
-CF2 |
=238 |
[(CF=CF)2N(C2F4)]+ |
0,2% |
|
238 |
-CF2 |
=188 |
[(CF=CF)2N(CF2)]+ |
0,2% |
|
188 |
-CF2 |
=138 |
[CF=CF-N-CF=CF]+ |
0,2% |
|
138 |
-C2F2 |
=76 |
[CF=N=CF]+ |
0,4% |
|
Ion current series 7,9 % (1,87 % of total ion current in the spectrum) |
||||
A comparison of perfluorotributylamine spectra recorded via a magnetic device and an ion trap device (Polaris Q) (Fig. 1), illustrates the conflict of series 1 and (1* with higher number of detachments), depending on the type of mass-spectrometer. The required conditions for reinforcement of series 1* is the higher excitation energy [M]+., and the longer period of fragmentation.
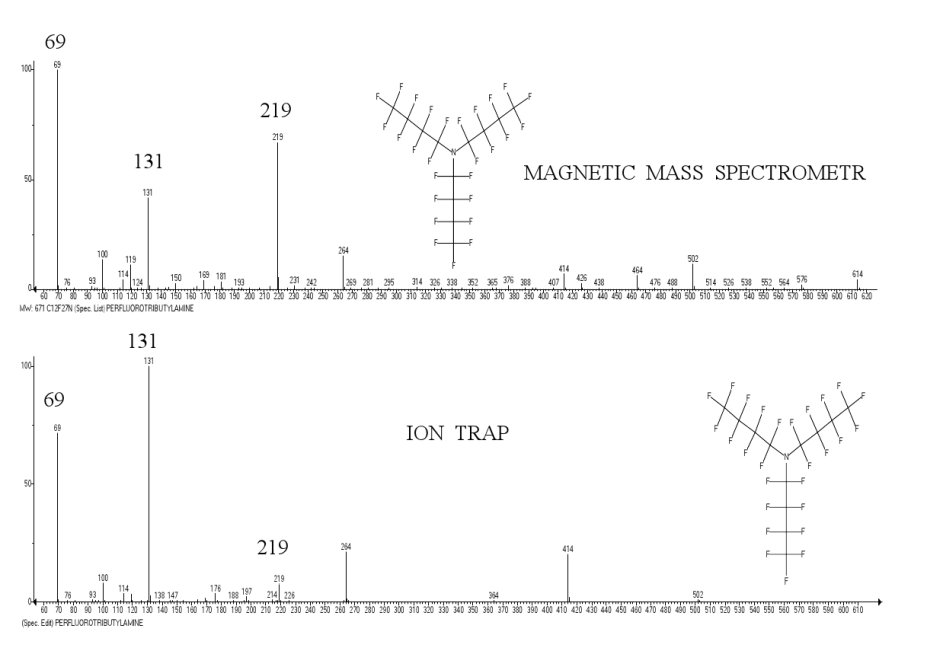
Figure. 1. Spectra of perfluorobutylamine (PFTBA), taken via a magnetic device and an ion trap.
If in the spectrum obtained via a magnetic device the alkyl fragmentation with base ion CF3 is the principal one, then in the conditions of an ion trap, as a result of additional stabilizing detachments of fluorine atoms, the alkenyl fragmentation with base ion [CF = CF-CF2]+ m/z 131 becomes the dominant.
In PFTBA spectrum, as well as in the spectra of n-alkanes and n-perfluoroalkanes [5], taken via devices with ion traps, an alkenyl series of ions is dominant. The reason for this, are the design features of the ion trap.
Conclusions
The three main series of PFTBA ions, occur during the decay of three concurrently formed molecular ions, with cation-radicals centers on (C4F9)2N. +CF2-α, and (C4F9)2NСF2+ .CF2-β σ-bonds of atoms central group in the molecule, as well as σ-bonds F. +CF2(CF2)3N(C4F9)2 of one of peripheral groups CF3.
Since the simultaneous detachment of three fluorine atoms from three terminal CF3 groups is unbelievable, we can conclude, that the detachment of one F atom from one of the CF3 groups initiates symmetrical detachments of two fluorine atoms from the other two groups.
The relative probabilities of PFTBA fragmentation pathways, presented in Table 1, correspond to the total ion current values of the three main series N1-3 and the three subseries N1*, N3*, and 3**.
Table 1. The ion current of PFTBA series, the relative probability of their formation, the total number of detachments, the total detached mass and the mass of final recorded ion.
|
N series |
N1 |
N1* |
N2 |
N3 |
N3* |
N3** |
|
Ion series current, % |
46,9 |
26,5 |
11,4 |
9,9 |
3,4 |
1,9 |
|
Relates series probabilities |
25,1 |
14,2 |
6,1 |
5,3 |
1,8 |
1,0 |
|
∑ Number of breaks |
4 2 |
5 |
10 |
13 |
15 |
16 |
|
∑ Tear off mass |
602 |
590 |
557 |
557 |
595 |
595 |
|
Mass of the final ion |
69 [CF3]+ |
81 [CF2=CF]+ |
114 [N(CF2)2]+ |
114 [N(CF2)2]+ |
76 [CF=N=CF]+ |
76 [CF=N=CF]+ |
In series N1, four radical detachments lead to the formation of alkyl ions, and two additional detachments lead to the formation of olefin ions [C3F6]+ and [C2F4]+.
As it can be seen from Table 1, the relative probabilities of series formation decreases in proportion to increase in the number of breaks occurring.
In comparison to the other series with the minimal number of detachments, the required condition for formation of series with maximal number of detachments, is the maximal excess energy [M]+..
As a result of two additional symmetrical detachments of fluorine atoms in the N1 series, as well as two or four additional detachments of fluorine atoms in the N3 series, alkenyl subseries of N1*, N3* and N3** ions are formed, the occurrence of which is a result of additional stabilization of primary ions of series N1 and N3. The probability of formation the N1* series formation, increases in devices with an ion trap, in case of extended presence of ions at the separation zone.
Separations of three, five and seven fluorine atoms, subsequent emissions of CF2, mass values of intermediate (CF2)3N and final CF2-N-CF2, CF = N = CF nitrogen-containing ions, allow us to conclude that perfluorotributylamine fragmentation occurs by symmetric detachments of fluorine atoms and emission of CF2 groups, with the preferred conservation of the symmetry of the formed ions.
Tertiary perfluoroamines with three substituents of different masses, are fragmented similarly to PFTBA, however, the sequence of detachments depends on the mass of substituents. The rule is, that the detachment of a nitrogen-containing radical .NR"R"', with the lowest mass substituents, leads to formation of a perfluoroalkyl ion [R']+ with the maximum mass of the substituent, and the separation of the maximum mass substituent R' without one CF2 group, that is, the radical .[R' -CF2] to the formation of the nitrogen-containing ion [R"R"'NCF2]+ with substituents having minimal masses. Thus, the fragmentation of tertiary perfluoroamines with three substituents of different masses also shows a tendency towards an increase in the symmetrization of the formed ions.
Acknowledgments
This work was supported by Ministry of Science and Higher Education of Russian Federation using scientific equipment of Molecules Structure Study Center of INEOS RAS.
Literature
- Iida Yoshi, Okada Shizuko, Seikei Daigaku Kogakubu Hokoku, 1984, 37, 2453-2454.
- Brilis, George Michael, Brumley, William C, Analytica Chemica Acta, 1990, 229(2), 163-8.
- Hvistendahl G., Undheim K., Org. Mass. Spectrom., 1970, 3, 821.
- Zahorszky, U.I., Organic Mass Spectrometry, 1979, 14(2), 66-74.
- Kagramanov N.D., Fluorine notes, 2020, 1 (128), 3-4.
ARTICLE INFO
Received 22 April 2020
Accepted 21 May 2020
Available online June 2020
Recommended for publication by Prof. S. Igumnov
Fluorine Notes, 2020, 130, 1-2
