Received: June 2020
DOI 10.17677/fn20714807.2020.03.03
Fluorine Notes, 2020, 130, 5-6
SYNTHESIS OF 4-CF3-SUBSTITUTED MONO- AND DICHLOROPHENYLSULFONYL CHLORIDES
T.P. Vasilieva*, V.I. Dyachenko
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: vic-d.60@mail.ru
Abstract: Using the available 4-CF3-substituted mono- and dichloroarylamines 1a, 4, the corresponding sulfonyl chlorides 3a and 6 were prepared from the Meerwein reaction with high yield. The key stage is the copper (I) catalyzed reaction of diazonium salts with SO2 in hydrochloric acid aqueous solution. Preparative methods for synthesis of sulfonyl chlorides 3a, b, and 6, which are highly scalable, have been developed.
Keywords: N-chlorosuccinimide, 2,6-dichloro-4-trifluoromethylaniline, diazotization, copper (I) chloride, 4-CF3-containing phenylsulfonyl chlorides
Arylsulfonyl chlorides are one of the most important classes of organic compounds. Many of them have found application in production of antibacterial and disinfectants, polymers, bleaches, herbicides and a number of other substances widely used in industry, medicine and agriculture [1, 2]. Despite the advent of antibiotics, arylsulfonylchloride-based sulfanilamide drugs have not yet lost their value and are successfully used in the therapy of infectious diseases [3]. This is especially true in case of antibiotic resistance progression in severe septic complications. In addition, the presence of fluorine and CF3-group atoms in the molecules of antibacterial and other biologically active compounds leads to an increase in their lipophilicity, improvement of pharmacokinetic properties, and a decrease in therapeutic doses of the drug [4, 5].
To date there have been many publications about possibility of using fluorine-containing arylsulfonyl chlorides not only to obtain antibacterial substances, but also in synthesis of sulfanilamide derivatives of synephrine (possessing specific induction of apoptosis [6]), compounds with high antitumor activity [7], substances lowering the blood sugar level (BSL) [8]. Using gamma secretase inhibitors the fluorine sulfonyl chlorides [9], bradykinin B1 receptor blockers [10] and oxytocin receptor antagonists were synthesized for autism and memory loss treatment [11]. 4-Fluorophenyl sulfamides of (S)-tryptamine have been proposed as anticancer agents [12], and fluorine-containing sulfamides of heterocyclic compounds have been recommended for treatment of cognitive impairment associated with Alzheimer's and Parkinson's disease [13].
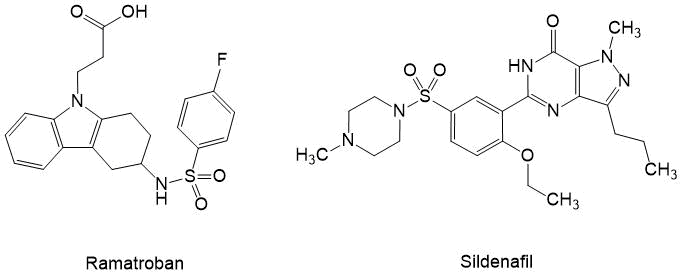
Figure 1.
A highly effective platelet antiaggregants, obtained on the basis of aryl sulfonyl chlorides have proven Ramatroban [14] and Sildenafil, having the ability to remove small vessel spasms, lungs and other organs [15] (see Fig. 1). This is especially important now that it has become known that one of the main causes of death by coronavirus infection COVID-19 (caused by the SARS-CoV-2 virus) is thrombosis and narrowing of alveoli lumen.
In this regard, the search for effective methods for synthesis of fluorine-containing arylsulfonyl chlorides as building blocks for organic and pharmaceutical chemistry is a vital task.
The aim of this work is to search and develop of effective methods for synthesis of 4-CF3-containing benzenesulfonyl chlorides - 2-chloro-4-(trifluoromethyl) benzenesulfonyl chloride (3a), 2,6-dichloro-4-(trifluoromethyl) benzenesulfonyl chloride (6) and also 2-chloro-4-cyanobenzenesulfonyl chloride (3b), widely used in organic synthesis [6–13]. In spite of the fact that sulfonyl chlorides 3a, 3b and 6 are often used to obtain biologically active substances, the methods for their synthesis are not described in special literature.
Traditional methods of sulfonylation of the corresponding benzenes using HSO3Cl, HSO3F, SO2Cl2, etc. [16,17], for the preparation of compounds 3a, 3b, 6 proved to be unpromising. This is due to both the inaccessibility for sulfonylation of the initial CF3-containing chlorobenzenes, and the high probability of obtaining a mixture of isomeric ortho- and para- reaction products.
In this regard, to develop acceptable methods for synthesis of 3a and 6 we used the Meerwein reaction, which consists in chlorosulfonylation of diazonium salts in a hydrochloric acid aqueous solution of SO2 in the presence of copper salts (I) [18], (see Scheme 1).
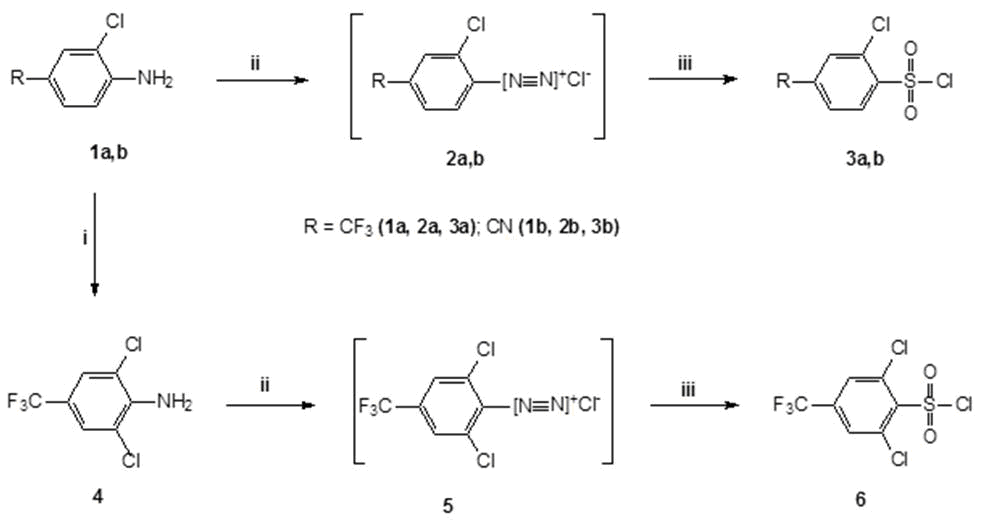
Sheme 1. Reagents and conditions: i) N- chlorosuccinimide, МеCN, 60-85С; ii) NaNO2, HCl/H2O, -5С, iii) SOCl2/H2O, 7-18С, 1-2 mol % CuCl, 0С.
The choice of this method for synthesis of desired sulfonyl chlorides was supported by availability of corresponding starting CF3-containing arylamines 1a, b and 4, as well as by previously described specific example of obtaining 2-chloro-4-cyanobenzenesulfonyl chloride under these conditions [19]. Unfortunately, this patent does not describe either a method for synthesizing the initial arylamine 1b or a physical characteristics of resulting sulfonyl chloride 3b. At the same time, the electron-accepting properties of CF3 - and CN-groups, comparable in their effect on the aromatic nucleus, made it possible to hold out a hope of successful application of this synthesis method of fluorine-containing sulfonyl chlorides 3a and 6.
Initial halogen-containing arylamines 1b and 4 were obtained by chlorination of commercially available 4-aminobenzonitrile and 2-chloro-4-(trifluoromethyl)aniline 1a with N‑chlorosuccinimide, as described in [20]. The known method of preparation 1b by reduction of 3-chloro-4-nitrobenzonitrile [21] more difficult.
It has been found that the optimum conditions for the chlorination of these arylamine N-chlorosuccinimide is to carry out the reaction in acetonitrile at 60 -85°C over 2 -3 hours. In this case, 4-amino-3-chlorobenzonitrile 1b is formed with a yield of 92%. Similarly, 2,6-dichloro-4-trifluoromethylaniline 4 was obtained with a yield of about 93% (see Scheme 1). Previously, the patent [22] reported the preparation of compound 4 by hydrolysis of 4-trifluoromethyl-2,6-dichlorophenyl isocyanate, however, neither the synthesis strategy nor the physical characteristics 4 were given in this work.
We synthesized sulfonyl chlorides 3a, b and 6 in three stages: 1) in situ generation of sulfur dioxide from SOCl2 with H2O; 2) preparation of diazonium salts from corresponding arylamines and NaNO2 in a hydrochloric acid aqueous solution 3) reacting of these diazonium chlorides salts 2a, b and 5 with SO2 under these conditions in the presence of copper chloride (I) (see Scheme 1). As it turned out in the case of arylamines containing hydrophobic substituents, the chlorosulfonylation reaction has specific features. Thus, upon preparation of diazonium salts 2a and 5, the intermediate-formed arylamine hydrochlorides 1a and 4, just like the diazonium chlorides formed from them, precipitate under typical conditions of Meyervain reaction. This leads to the fact that diazotization is performed under heterogeneous conditions, which significantly reduces the reaction control and the yield of final products. The poor solubility of salts 1a, 4 and 2a, 5 in an aqueous medium is obviously due to the presence in structure their molecules (in addition to chlorine atoms) a highly hydrophobic CF3-group. In this regard, the diazotization of these arylamines we did not performed in a hydrochloric acid aqueous solution, but in H2O-HCl-AcOH system. Indeed, the use of a small amount of AcOH as a solvent in these transformations significantly affects the solubility of initial and intermediate reaction products. In turn, this leads to an improvement in formation of target compounds. Thus, the sulfonyl chlorides 3a and 6 were obtained with a yield of 71% and 76%, respectively.
Apparently, this approach to synthesis of 3a and 6 can be successfully used to obtain other polyfluoro- and polyfluoroalkyl-containing sulfonyl chlorides.
Experimental part
1H and 19F NMR spectra were recorded in CDCl3 via Bruker Avance-400 spectrometer with operating frequencies of 400 MHz and 376 MHz, respectively. The chemical shifts in 1H NMR spectra of obtained compounds are given on a scale of δ (ppm) relative to TMS (internal standard), 19F -relative to CFCl3 (external standard). The spin-spin interaction constants are given in Hz. Rf of obtained compounds were determined by TLC method using Merck plates (Silica gel 60 F254) in the system hexane-chloroform = 3: 1.
Initial 4-aminobenzonitrile (CAS 873-74-5) and 2-chloro-4-trifluoromethylaniline (CAS 21803-75-8) - from Sigma-Aldrich. Elemental analysis of obtained compounds was carried out in the laboratory of elemental analysis of INEOS RAS.
2-Chloro-4-trifluoromethylbenzenesulfonyl chloride (3a)
Stage A. Obtaining a solution of SO2 in dilute hydrochloric acid. In a three-necked two-liter round-bottom flask equipped with a magnetic stirrer and a thermometer 522 ml of Н2О were placed and cooled with ice to 0-7 °С. Under these conditions, into this flask was added dropwise 88 ml (1,205 mol) of SOCl2 for 1 hour with stirring.
Stage B. Obtaining a solution of diazonium hydrochloric salt 2a. In a three-necked half-liter round-bottom flask equipped with a thermometer and a magnetic stirrer, 190 ml of concentrated HCl and 38 ml of AcOH were placed. To the resulting acid mixture a solution of 55 g (0,28 mol) of arylamine (1a) in 35 ml of AcOH was added with stirring, then the temperature of reaction mixture was lowered to -10-5 °C and with vigorous stirring a solution of 20,9 g (0,3 mol) of NaNO2 in 83 ml of water was added dropwise. After stirring at -5-0 °C for 1 hour, the reaction mixture was transferred into a dropping funnel to carry out the final stage of the reaction
Stage C. In a two-liter flask containing the resulting SO2 solution in dilute hydrochloric acid (see Stage A), 0.5 g (5,06 mmol) of copper chloride (I) was added. The temperature of reaction mass was reduced to 0-7 °C with stirring, and then a cold (0-5 °C) solution of diazonium 2a hydrochloride salt was added to it from a dropping funnel (see Stage B). After stirring the reaction mass at 0°C for 1,5 hours, the cooling was stopped, and the reaction temperature was brought to 18°C. To isolate the reaction product, 150 ml of CCl4 was added to reaction mixture, stirred for 5 minutes, and the organic layer was separated. The aqueous layer was extracted at first with 100 ml of benzene, and then - with 150 ml of CH2Cl2. The combined organic solutions was washed with cold water, dried over MgSO4, the solvent was removed, and the residue was was distilled in vacuo. 55,4 g (71%) of sulfonyl chloride (3a) was obtained as a pale yellow oil having a boiling point 88‑89°C at 1,5 mm Hg. On standing the substance 3a becomes solid, forming transparent crystals with a melting point of 38-40°C. Its spectral purity is 97%, Rf = 0,39 (CCl4).
1H NMR (CDCl3, δ, ppm, J/Hz): 7,78 (d, 1H, Ar, 3JH-H = 8.4); 7,92 (s, 1H, Ar); 8,31 (d, 1H, Ar, 3JH-H = 8.4.
19F NMR (CDCl3, δ, ppm, J /Hz): -63,47 (s, 3F, CF3).
Found: C 30,03; H, 1,02; F, 20,20. C7H3Cl2F3O2S. Calculated: C, 30,11; H, 1,08; F, 20,43.
4-Amino-3-chlorobenzonitrile (1b)
To a solution of 50 g (0,423 mol) of 4-aminobenzonitrile in 420 ml of MeCN at a temperature of 60 °C, 56,51 g (0.423 mol) of N-chlorosuccinimide were added in portions. Then the reaction temperature was raised to 85 °C and stirred under these conditions for 3 hours. After that, the reaction mass was evaporated on a rotary evaporator to half of volume, and then into remaining solution 390 ml of a 5% aqueous solution of NaOH was poured with vigorous stirring. The precipitated light yellow solid precipitate was filtered off, washed with water, petroleum ether, and then dried over KOH to a constant weight at 20 mm Hg. Yield 59.3 g (92 %) of compound 1 with a melting point 103-104 °C (in [20] melting point equal 97-99 °C).
1H NMR (CDCl3, δ, ppm, J /Hz): 4,53 (br s, 2H, NH2); 6,76 (d, 1H, Ar, 3JH-H = 8.4), 7,33 (br d, 1H, Ar, 3JH-H = 8,4); 7,52 (br s, 1H, Ar).
According to TLC Rf = 0,40 (CHCl3), and 1H NMR, the purity of compound was 98.5%.
2-Chloro-4-cyanobenzenesulfonyl chloride (3b)
Prepared analogously to the synthesis of 3a. In a three-necked two-liter round-bottom flask equipped with a magnetic stirrer and a thermometer, 528 ml of Н2О were placed and cooled with ice to 0-7 °С. Under these conditions, 88,6 ml (1,23 mol) of SOCl2, then 0,319 g (3,22 mmol) of CuCl were added to the flask from a dropping funnel with stirring for 1 hour. After this, a solution of diazonium salt 2b obtained by mixing solutions of 43,32 g (0.284 mol) of arylamine 1b in 285 ml of concentrated HCl and 21,1 g (0,306 mol) of NaNO2 in 84 ml of water was added from a dropping funnel to reaction mass upon cooling to 0-5°C. After stirring the reaction mass under these conditions for 1,5 hours, cooling was stopped, and the reaction temperature was brought to 18°C. The resulting precipitate was filtered off and washed with cold water. The crude product was extracted with 400 ml of methylene chloride, and the resulting solution was passed through a layer of silica gel. The filtrate was evaporated to half of volume, and 77 ml of CCl4 was added to it. The resulting solution was evaporated on a rotary evaporator until crystallization and cooled.
The precipitate formed was filtered off, washed with cold CCl4 and dried in vacuo over P2O5 at 20 mm Hg. Yield 44,3 g (66%) of spectrally pure sulfonyl chloride 3b in form of a pale yellow crystals with melting point 102-103°C, Rf = 0.57 (chloroform).
1H NMR (CDCl3, δ, ppm, J /Hz): 7,81 (d, 1H, Ar, 3JH-H = 8,4); 7,95 (s, 1H, Ar); 8,29 (d, 1H, Ar, 3JH-H = 8,4).
Found, %: C, 35,73; H, 1,28; Cl, 30,04; N, 5,91. C7H3Cl2NO2S. Calculated,%: C, 35,59; H, 1,27; Cl, 30,08; N, 5,93.
2,6-Dichloro-4-trifluoromethylaniline (4)
Prepared in acetonitrile from 107,71 g (0,551 mol) of 2-chloro-4-trifluoromethylaniline 1a and 80,91 (0,606 mol) of N-chlorosuccinimide analogously to the synthesis of compound 1b for 2 hours. The solvent was removed in vacuo, and to the residue was added 200 ml of petroleum ether. The precipitated succinimide was filtered off and the filtrate was evaporated on a rotary evaporator. The remaining oil was distilled in oil pump vacuo, obtaining 4 as a pale yellow oil with a boiling point of 61-62 °C at 1-2 mm Hg. with a yield of 93%, Rf = 0,75 (CCl4-CHCl3 = 1: 1). After cooling, 4 turns into a solid with a melting point of 33-35 °C (in [22] melting point equal 32-35 °C).
1H NMR (СDСl3, δ, ppm, J /Hz): 4,80 (br s, 2H, NH2); 7,50 (s, 2H, Ar).
19F NMR spectrum (СDСl3, δ, ppm, J /Hz): -61,97 (s, 3F, CF3).
Found, %: C, 36,27; H, 1,65; F, 24,71; N, 5,98. C7H4Cl2F3N. Calculated, %: C, 36,52; H 1,74; F, 24,78; N, 6,09.
2,6-Dichloro-4-trifluoromethylbenzenesulfonyl chloride (6)
Prepared analogously to the synthesis of 3a. In a two-liter, three-necked round-bottom flask equipped with a magnetic stirrer and a thermometer, 429 ml of Н2О were placed, cooled to 0-7 °С and 72 ml (0.986 mol) of SOCl2 were added to the flask for 1 hour, then 0.45 g ( 4.55 mmol) of copper chloride (I). Then, while cooling to 0-5°C, a solution of diazonium salt 5 was added from a dropping funnel over a period of 1.5 h. In turn, 5 was prepared by adding a mixture of 232 ml concentrated HCI and 35 ml ACOH first solution 53.17 g (0.231 mol) of aryl amine 4 in 35 ml Acoh, then 17.2 g (0.249 mol) of NaNO2 in 69 ml of water. After stirring at 0-5°C over 1.5 hours, cooling was removed, the temperature of the reaction mass was adjusted to 18°C. The precipitate was filtered off, washed with cold water and dried in vacuo over Р2О5 at 15 mm Hg. Yield 55,12 g (76%) of spectrally pure sulfonyl chloride 6 in form of yellow crystals with melting point 48-50 °C, Rf = 0,42 in CCl4, purity ≥ 98%.
1H NMR (CDCl3, δ, ppm, J /Hz): 7.82 (s, 2H, Ar).
19F NMR (CDCl3, δ, ppm, J /Hz): -61,32 (s, 3F, CF3).
Found: C, 26,77; H, 0,64; F, 18,18. С7Н2Cl3F3O2S. Calculated: C, 26,79; H, 0,61; F, 18,05.
Conclusions
It was shown that para-CF3-substituted arylamines containing one or two chlorine atoms in the ortho-position easily interact under the conditions of Meerwein reaction with SO2, forming the corresponding sulfonyl chlorides 3a, 6 with a high yield.
Preparative methods have been developed for obtaining of sulfonyl chlorides 3a, b and 6, which are widely in demand as building blocks in processes of synthesis of biologically active substances. The prospects of scaling these methods at practice are studied.
Acknowledgments
This work was financially supported by the Ministry of Science and Higher Education of Russian Federation using scientific equipment of the Center of Molecules Structure Study of INEOS RAS.
Literature
- Gilbert E. E., Sulfonation of organic compounds, trans. from English., M., 1969.
- Houben-Weyl, Methoden der organischen Chemie, Bd 9, Stuttg., 1955.
- In the book S. N. Kozlov, R. S. Kozlov, Modern Antimicrobial Chemotherapy: A Guide for Physicians, 3rd ed. Publishing House “Medical Information Agency Russia”, Article: 108458, ISBN: 978-5-8948-1999-0, 2017, 400 pp.
- H-J. Böhm, D. Banner, St. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander, M. Stahl, ChemBioChem, 2004, 5, 637.
- T. Hiyama, Organofluorine Compounds, Springer Berlin Heidelberg, Berlin, Heidelberg, 2000.
- Vanden Berghe, Wim; De Bosscher, Karolien; Van Calenbergh, Serge; Haegeman, Guy; Lacey, Carl Jeffrey; Gossye, Valerie; Arias, Ruben Hoya; Gerlo, Sarah /Patent US 20090029999, 2009.
- Bystroem, Styrbjoern; Hedgecock, Charles; Homan, Evert; Lundbaeck, Thomas; Martinsson, Jessica; Sari, Meral; Faernegaardh, Katarina; Joensson, Mattias / WO 2012035171, 2012.
- Liang, Yanshu; Zhu,Yingjie; Guo, Guimei; Lu, Liang; Long, Li / Faming Zhuanli Shenqing, Patent CN 103012314, 2013.
- Adeniji, A. O.; Wells, R. M.; Adejare, A. Current Medicinal Chemistry, 2012, 19(15), 2458‑2471.
- Reich, Melanie; Schunk, Stefan; Jostock, Ruth; Hees, Sabine; Germann, Tieno; Engels, Michael Franz-Martin, WO 2010051977, 2010.
- Bissantz, Caterina; Grundschober, Christophe; Nettekoven, Matthias; Plancher, Jean-Marc; Vifian, Walter, WO 2014111356, 2014.
- Guo, Zhenbo; Xu, Yiming; Peng, Yujie; Haroon ur Rashid; Quan, Wei; Xie, Peng; Wu, Lichuan; Jiang, Jun; Wang, Lisheng; Liu, Xu, Bioorganic & Medicinal Chemistry Letters, 2019, 29(9), 1133-1137.
- Chu, Chester; Lister, Andrew; Nordvall, Gunnar; Petersson, Carl; Rotticci, Didier; Sohn, Daniel, WO 2006126939, 2006.
- Motobayashi Y., Imagawa W., Saida K. Ramatroban (Baynas): a review of its pharmacological and clinical profile, Folia Pharmacol. Jpn., 2001, 118(6), 397-402.
- Shmal'ts A.A., Gorbachevskiy S.V. Riociguat and sildenafil for pulmonary hypertension: similarity and difference, Pulmonologiya, 2016, 26(1), 85-91. (In Russian) https://doi.org/10.18093/0869-0189-2016-26-1-85-91.
- Sykes P., Mechanisms of reactions in organic chemistry / Trans. from English by N. G. Lutsenko; Ed. V. F. Travenya. 4th ed. M.: Chemistry,, 1991, 446p., ISBN 5-7245-0191-0.
- Titze L., Eicher T., Preparative organic chemistry: Trans. from Deutsch / Ed. Yu.E. Alekseeva. M .: Mir, 1999, 704p.
- Meerwein H. et al. Reaction of aromatic diazo compounds upon α,β-unsaturated carbonyl compounds, Journal fur Praktische Chemie (Leipzig), 1939, 152, 237-266.
- Fritson I., Liberg D., East S., Mackinnon C., Prevost N., WO 2014184234, 2014.
- Nicson T.E., Roche-Dalson C.A., Synthesis, 1985, 669.
- Grivsky E/M., Hitchings G.H., Ind. Chim. Belge, 1974, 39, 490.
- Fаerbenfabriken BayerA.-G, Patent FR 1545142, 1968.
ARTICLE INFO
Received 15 June 2020
Accepted 16 June 2020
Available online June 2020
Recommended for publication by Prof. S. Igumnov
Fluorine Notes, 2020, 130, 5-6
