Received: July 2020
DOI 10.17677/fn20714807.2020.04.01
Fluorine Notes, 2020, 131, 1-2
A NOVEL REDUCTION REACTION FOR THE CONVERSION OF TRIBUTYL(TRIFLUOROMETHYL)STANNANE INTO TRIBUTYL(DIFLUOROMETHYL)STANNANЕ
Alexander S. Golubev, Petr N. Ostapchuk
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov St. 28, 119991, GSP-1, Moscow, Russian Federation
E-mail: golubev@ineos.ac.ru
Abstract: A new method for obtaining tributyl(difluoromethyl)stannane n-Вu3SnCF2H by reduction of n-Вu3SnCF3 with lithium borohydride in diglyme has been developed.
Keywords: tributyl(difluoromethyl)stannanе, tributyl(trifluoromethyl)stannane, lithium borohydride, reduction.
The introduction of a difluoromethyl group into organic molecules is often used in the development of new pharmaceuticals and agrochemicals [1]. Several years ago, a method for difluoromethylation of aryl, heteroaryl and β-styryl iodides using tributyl(difluoromethyl)stannane n-Bu3SnCF2H was developed. The synthesis of n-Bu3SnCF2H was accomplished by the reaction of tributyltin hydride with trifluoromethyltrimethylsilane Me3SiCF3, with column chromatography being used to purify n-Bu3SnCF2H [2].
As part of an ongoing project on the synthesis of difluoromethyl-containing physiologically active compounds, we needed n-Вu3SnCF2H free of n-Bu3SnCF3. In experiments on the synthesis of n-Bu3SnCF2H according to the protocol given in [2], we have always observed the formation of tributyl(trifluoromethyl)stannane n-Bu3SnCF3 as a by-product. Column chromatography could indeed significantly reduce the content of n-Bu3SnCF3 impurity. Nevertheless, it was possible to completely get rid of n-Вu3SnCF3 only at the expense of a significant loss of the target n-Вu3SnCF2H. To obtain n-Вu3SnCF2H not containing n-Вu3SnCF3, we have developed a new reduction reaction for the conversion of n-Вu3SnCF3 to n-Вu3SnCF2H.
We found that tributyl(trifluoromethyl)stannane n-Вu3SnCF3 reacted with lithium borohydride LiBH4 in diglyme to form tributyl(difluoromethyl)stannane n-Вu3SnCF2H (scheme 1).
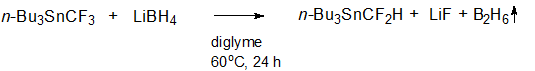
Scheme 1.
Application of 1.5 equivalents of LiBH4 and moderate heating (60 °C) brought about a complete conversion of the starting n-Вu3SnCF3 and a good yield of the target n-Вu3SnCF2H (60-70%) in a reasonable time (24 h). The formation of n-Вu3SnCF2H was also observed at room temperature, but the conversion did not exceed 50% in 24 hours. The completion of the reaction was monitored by 19F{1H} NMR spectroscopy (n-Вu3SnCF3 showed a singlet at -45.13 ppm with 119Sn satellites: 2J (19F,119Sn) = 214 Hz; n-Вu3SnCF2H showed a singlet at -124.62 ppm with 119Sn satellites: 2J (19F,119Sn) = 212 Hz) and 119Sn NMR spectroscopy (a quartet with at -22.7 ppm with a coupling constant 2J (19F,119Sn) = 218 Hz for n-Вu3SnCF3; a triplet at -49.4 ppm with a coupling constant 2J (19F,119Sn) = 214 Hz for n-Вu3SnCF2H).
The main byproduct of the reaction was bis(tributyltin) oxide. Its formation apparently occurs due to traces of water in the reagents (LiBH4 is extremely hygroscopic).
Only one hydride ion of the LiBH4 molecule was involved in the reduction reaction. Since diborane was released during the reaction, the reduction process had to be carried out under a continuous flow of nitrogen flow. The gas stream leaving the reaction vessel was bubbled through a column of water or acetone.
By the end of the reaction, clear partitioning of the reaction solution into two phases was observed. The study of the phases showed that n-Вu3SnCF2H was almost completely in the lower phase. Unreacted LiBH4 and the remaining diborane were in the upper phase. Their interaction, as is known [3,4], leads to the formation of LiB2H7. Lithium diborohydride binds diglyme (DG) in the form of a solvate complex of the LiB2H72DG structure, which was apparently the reason for partitioning of the reaction solution.
Treatment of the reaction with water required special attention. It is recommended to pour the reaction into ice water. Adding water to the reaction in one of the experiments caused a vigorous reaction, which led to the splashing of the contents of the reaction flask.
Our attempts to use sodium borohydride NaBH4 in diglyme for the reduction of n-Вu3SnCF3 by analogy with the reduction of trifluoromethyltrimethylsilane Me3SiCF3 to difluoromethyltrimethylsilane Me3SiCF2H [5] met no success. We observed the formation of n-Вu3SnCF2H, however, we failed to achieve a significant conversion in the temperature range of 20-50 °C. Interestingly, when reducing Me3SiCF3 to Me3SiCF2H with sodium borohydride, at least 3 hydride ions of the NaBH4 molecule are involved in the process [5].
Thus, we have developed a new method for obtaining tributyl(difluoromethyl)stannane
n-Вu3SnCF2H
by reduction of n-Вu3SnCF3 with lithium borohydride in diglyme. The
new method avoids the use of unsafe and prone to oxidation tributyltin hydride. An advantage of the
method is the possibility of obtaining Вu3SnCF2H that does not contain n-Вu3SnCF3.
Experimental part
1H, 19F, 119Sn NMR spectra were recorded on a Bruker AvanceTM400 spectrometer (400.13 MHz for 1H, 376.50 MHz with proton decoupling for 19F, 149.21 MHz for 119Sn). The proton chemical shifts were measured relative to the residual solvent signal (δ (CDCl3) 7.28 ppm) and recalculated from the SiMe4 signal. The 19F NMR chemical shifts were measured relative to trifluoroacetic acid (an internal standard) and referenced to CFCl3. 119Sn NMR chemical shifts were determined relative to Me4Sn as an internal standard (δ 0.0 ppm).
Tributyl(trifluoromethyl)stannane n-Вu3SnCF3 was prepared from bis(tributyltin) oxide and trifluoromethyltrimethylsilane Me3SiCF3 according to the procedure described in [6].
The reaction was carried out in a 3-necked round bottom flask under a stream of dry nitrogen. The flask was equipped with a dry nitrogen inlet tube, a dropping funnel and an outlet connected to an absorption column with water or acetone. To a solution of 7.0 g (19.5 mol) tributyl(trifluoromethyl)stannane in 30 ml of dry diglyme was added lithium borohydride (0.64 g, 29.3 mmol) as a solution in diglyme (9.8 ml of a 3M solution in diglyme). The addition of LiBH4 was accompanied by a rapid rise in the temperature of the reaction mixture to 35 °C. The reaction mixture was heated with stirring at 60 °C for 24 hours. Reaction control (19F NMR) showed complete conversion of the starting n-Вu3SnCF3. The reaction was poured into ice water (200 ml), extracted with toluene (50 ml + 30 ml). The combined organic phases were evaporated in vacuo on a rotary evaporator. The residue was distilled, and 4.2 g (63%) of the liquid with b.p. 89-93 °C/0.3 Torr was obtained, which was tributyl(difluoromethyl)stannane n-Вu3SnCF2H and about 6 mol. % diglyme. Tributyl(difluoromethyl)stannane n-Вu3SnCF2H can be further purified through a small pad of silica gel, eluent petroleum ether: ethyl acetate 100: 1, to detect cerium molybdate (Hanessian's Stain).
1H NMR (CDCl3) δ 6.45 (t, J = 44.9 Hz, 1H), 1.70 – 1.44 (m, 6H), 1.44 – 1.24 (m, 6H), 1.21 – 0.97 (m, 6H), 0.93 (t, J = 7.3 Hz, 9H).
19F{1H} NMR (CDCl3) δ -124.62 s with 119Sn satellites: 2J (19F,119Sn) = 212 Hz).
119Sn NMR (CDCl3) δ -51.94 (t, J = 214.4 Hz) (see, also [2]).
Acknowledgments
This work was performed with the financial support from Ministry of Science and higher Education of the Russian Federation using the equipment of Center for molecular composition studies of INEOS RAS.
Literature
- Yerien D. E., Barata‐Vallejo S., Postigo A., Difluoromethylation reactions of organic compounds, Chemistry–A European J., 2017, 23(59), 14676-14701.
- Prakash G. K. S., Ganesh S. K., Jones J.-P., Kulkarni A., Masood K., Swabeck J. K., Olah G. A. Copper‐mediated difluoromethylation of (hetero)aryl iodides and β‐styryl halides with tributyl(difluoromethyl)stannane, Angewandte Chemie, 2012, 124(48), 12256-12260.
- Brown H. C., Stehle P. F., Tierney P. A., Singly-bridged compounds of the boron halides and boron hydrides., J. Am. Chem. Soc., 1957, 79(8), 2020-2021.
- Titov L. V., Gavrilova L. A., Borohydrides of the alkali and alkaline-earth metals., Russ. Chem. Bull., 1976, 232-235.
- Tyutyunov A. A., Boyko V. E., Igoumnov S. M. The unusual reaction of (trifluoromethyl)-trimethylsilane with sodium borohydride., Fluorine Notes, 2011, Vol. 1(74).
- Prakash G. K. S., Yudin A. K., Deffieux D., Olah G. A., Synthetic methods, Part 197, Facile preparation of (trifluoromethyl)tributyltin and transtrifluoromethylation of disilyl sulfides to the corresponding trifluoromethylsilanes, Synlett, 1996, 2, 151-153.
ARTICLE INFO
Received 14 July 2020
Accepted 15 July 2020
Available online August 2020
Recommended for publication by Prof. S.M. Igumnov
Fluorine Notes, 2020, 131, 1-2
