Received: February 2021
DOI 10.17677/fn20714807.2021.01.02
Fluorine Notes, 2021, 134, 3-4
THREE-COMPONENT ONE-STEP SYNTHESIS OF NEW NITRILES 3-(1H-INDOLE-3-YL)-2-CYANO-4,4,4-TRIFLUOROBUTANOIC ACID
O. Yu. Fedorovskii, N. D. Chkanikov
Nesmeyanov Institute of Organoelement Compounds, RAS, Vavilova 28, Moscow, 119991 Russia
e-mail: offskii@rambler.ru
Abstract: A new highly electrophilic alkene – 2-(2,2,2-trifluoroethylidene)malononitrile 1 and nitriles of 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluorobutanoic acid 2a - f on its basis were synthesized. Compounds 2a, b were obtained by C3-alkylation of indole derivatives 4a, b by alkene 1 with high yields, which, however, do not compensate for the low yield of 1. To increase the common yield of target nitriles 2, a three-component one-step reaction of trifluoroacetaldehyde ethyl hemiacetal, malononitrile and 4c - f indole derivatives was developed. The maximum yields of 2c - f compounds are achieved when all the reagents are mixed simultaneously in the equimolar ratio and the catalytic amount of the organic base. The structure of the compounds was confirmed by 1H, 13C and 19F NMR spectroscopy, mass spectrometry, and high-resolution mass spectrometry.
Keywords: 2-(2,2,2-trifluoroethylidene)malononitrile, nitriles 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluorobutanoic acid, three-component one-step synthesis, trifluoroacetaldehyde ethyl hemiacetal, indole and its derivatives, malononitrile.
Introduction
For many years, we systematically studied fluorinated highly electrophilic dicyanoethylenes synthesized from polyfluorocarbonyl compounds and malononitrile. [1 - 5]. One of the substrates that under mild conditions regeoselectively undergo C-alkylation at 3d position by similar alkenes is indole and its derivatives. 3-Substituted indoles underlie the structures of many natural compounds, medicinal drugs, and plant growth regulators [5 - 7]. The synthesis of new fluorinated 3-substituted indoles expands the prospect of creating effective biologically active compounds. The classical scheme for the synthesis of fluorinated dicyanoethylenes involves the C-oxyalkylation of malononitrile with a fluorinated carbonyl compound in the presence of a base or Lewis acid, followed by dehydration of the resulting alcohol. This method was used to obtain dicyanoethylenes from polyfluorinated ketones and trifluoropyruvic acid esters [2, 4 - 6].
In this paper, we studied methods for the synthesis of new nitriles 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluorobutanoic acid 2a - f based on trifluoroacetaldehyde ethyl hemiacetal.
Results and discussion
The study of the reaction of trifluoroacetaldehyde ethyl hemiacetal (TFAE) with malononitrile in the
presence of catalytic amounts of triethylamine showed that the C-hydroxyalkylation product 3 is spontaneously dehydrated to form alkene 1. The presence of 1 in the reaction mass in an amount of ~ 5% is confirmed by 1H and 19F NMR spectroscopy
(Scheme 1). It was found that in the reaction mixture, alkene 1 is unstable and
enters into various reactions with the formation of unknown by-products. An attempt to increase the
yields of alkene 1 by changing the ratio of the starting compounds (TFAE and malononitrile)
did not lead to positive results. An increase of the reaction temperature and/or the contact time
of the reagents leads only to the tarring of the reaction mass. The use of other basic catalysts
instead of triethylamine, such as DABCO, pyridine, quinoline, K2CO3, in different
ratios, or the use of their mixtures with triethylamine, does not give the better results. Incomplete
conversion of two consecutive reversible reactions and their final product - highly reactive alkene
1, which easily enters into side irreversible reactions with the initial and/or
intermediate compounds, significantly complicate its synthesis. Nevertheless, high reactive dicyanoethylene
1 was isolated by distillation from the reaction mass with a yield of 7%.
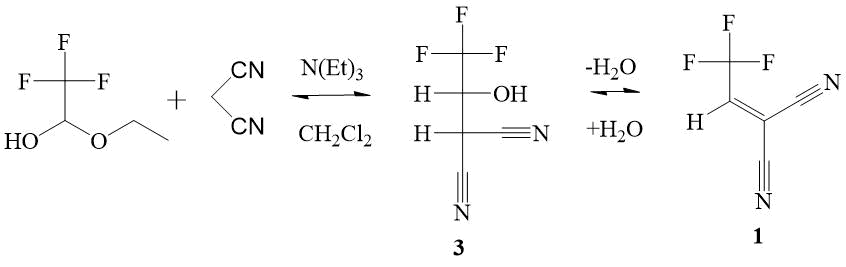
Scheme 1. Two-steps scheme of the synthesis of 2-(2,2,2-trifluoroethylidene)malononitrile 1. Under the conditions of synthesis, TFAE is gradually hydrolyzed by the released reaction water.
The reaction of indole and 2-methylindole with alkene 1 in dichloromethane leads to the almost quantitative formation of nitriles 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluorobutanoic acid 2a and b, which are isolated with yields of 92 % and 89 %, respectively. However, the low yield of alkene 1 stimulated the authors to search for new, alternative methods for the synthesis of target compounds 2.
The spontaneous formation of alkene 1 at the C-oxyalkylation stage allowed using 1 for C3-alkylation of indole 4 derivatives without prior it from the reaction mass. The addition of N-methylindole 4c to the reaction mass four hours after its preparation from equimolar amounts of TFAE, malononitrile, and a catalytic amount of triethylamine in dichloromethane resulted in the formation of compound 2с with a yield of 74 %.
It is known that TFAE reacts with indole only at high temperature or in the presence of Friedel - Сrafts
catalysts or under microwave irradiation [8 - 11]. At room temperature, under reaction conditions,
TFAE does not react with indole, and therefore cannot compete with alkene 1 in the
substitution reaction of indole 4 derivatives. Comparing the data from the literature
and our experiment, it became obvious that all the reagents can be mixed simultaneously. For comparison,
compound 2c was obtained a second time, but now all the reagents were mixed simultaneously.
In this one-step synthesis performance, compound 2c was isolated with a yield of
81%. This allowed in this work to implement the strategy of in situ synthesis of highly
reactive alkene 2-(2,2,2-trifluoroethylidene)malononitrile 1 in the presence of
indole 4 derivatives and take placed a three-component one-step reaction under basic
catalysis (Scheme 2). Compounds 2c - f are formed as a result of
a regeoselective electrophilic attack of intermediate 1 at position 3 of indole
4 derivatives, the formation of which in the reaction mass was confirmed by 1H
and 19F NMR spectroscopy.
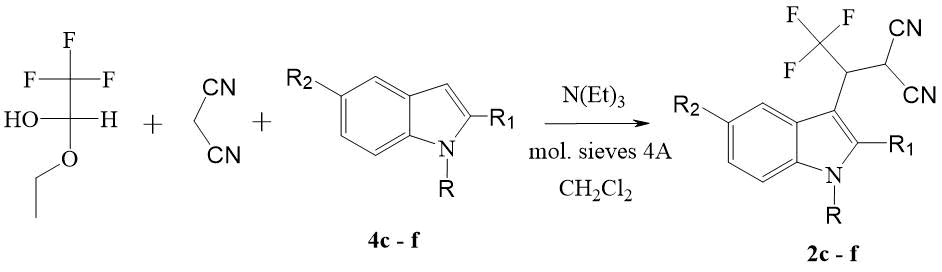
Scheme 2. Three-Component one-step synthesis of nitriles 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluorobutanoic acid 2c - f.
c) R=Me, R1=R2=H; d) R=Bnz, R1=R2=H; e) R=R1=H, R2=Br; f) R=R2=H, R1=Ph.
Maximum yields of 2c - f compounds (56 - 81 %) were achieved when equimolar amounts of TFAE, malononitrile, and 4c - f indole derivatives were mixed simultaneously in the presence of a catalytic amount of triethylamine at room temperature. The C3-alkylation reaction of indole derivatives 4c - f wins the competition with the side transformations of the alkene 1 formed in situ, providing high yields of the products of the main reaction-the target nitriles 2.
Thus, an effective method has been developed for the synthesis of new nitriles 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluorobutanoic acid 2, which are of great interest in the search for biologically active compounds. The use of a three-component one-step synthesis involving TFAE opens up new possibilities in the synthesis of trifluoromethyl-substituted compounds. Currently, one-step multicomponent reactions have become a valuable tool in modern organic synthesis for reducing stages, increasing the efficiency of synthesis methods, and reducing their cost [12, 13]. The maximum transformation of initial compounds into final products 2 (80 - 85 %) provides almost complete absence of chemical waste, that is, in this case, the principle of "atom economy" is implemented, which is one of the main principles for "green chemistry" [14].
Experimental part
All the original compounds were purchased from Merck and Sigma-Aldrich and used without further purification. All eluents and solvents: petroleum ether with tbp = 40-70°C (PE), EtOAc (EA), C6H6, DCM and NEt3 were purified by distillation before use. Merck Kieselgel 60 F254 TLC plates were used for reaction control and substance detection. The products were purified by column chromatography using Merck Kieselgel 60 silica gel (0.06-0.20 mm).
The 1H, 13C DEPT 135 and 19F NMR spectra were recorded on a Bruker AvanceTM400 spectrometer with operating frequencies of 400, 100, and 376 MHz, respectively. Chemical shifts of protons were determined relative to the residual CDCl3 signals (7.26 ppm) and recalculated to the SiMe4 signal. Chemical shifts of 19F NMR were determined with the suppression of the spin-spin coupling relative to trifluoroacetic acid as an external standard and recalculated to the CFCl3 signal.
Electron impact (70 eV) mass spectra were obtained with a Finnigan Polaris Q instrument equipped with an ion trap. The samples were introduced via either the direct inlet. The mass spectrum of compound 2e containing a bromine atom contains data for ions containing the 79Br isotope. Electrospray ionization (ESI) high resolution mass spectrometry was carried out with a Bruker micrOTOF II instrument operating in positive (capillary voltage of 4500 V) and negative (capillary voltage of 3200 V) ion modes. Operating mass range was m/z 50 - 3000 Da, calibration was either internal or external (Electrospray Calibrant Solution, Fluka). The samples were introduced via a syringe inlet as a solution in MeCN, MeOH, and water at flow rate of 3 μl*min–1. Nebulizer gas was nitrogen (flow rate of 4 l*min–1), inlet temperature was 180°C [15].
2-(2,2,2-Trifluoroethylidene)malononitrile (1).
The dichloromethane (5.0 ml), malononitrile (3.3 g, 0.05 mol), trifluoroacetaldehyde ethyl hemiacetal (7.2 g, 0.05 mol) and triethylamine (0.35 g) were added sequentially into a round-bottom flask with magnetic stir bar under the argon atmosphere. The reaction mixture was stirred at room temperature for overnight. When the reaction done, the solvent was removed under reduced pressure, the reaction mass was cooled and P2O5 (14.2 g, 0.1 mol) was added. A top-down refrigerator and a trap with dry ice are placed. The reaction mass was gently heated at a reduced pressure (~20 mm Hg). Alkene 1 after top-down refrigerator is collected in a trap with dry ice. Obtained transparent oil with a sharp irritating smell, 0.52 g, yield 7%, tbp 80-85C at ~ 20 mm Hg. The substance is tarred for a week at 5°C.
1Н NMR (CDCl3, δ, ppm., J/Hz): 7.13 (1Н, q, 6.3 Hz, СF3-СН=).
19F NMR (CDCl3, δ, ppm.): -62.91 (s, -CF3).
MS (EI, 70 eV), m/z (Irel (%)): 146 [М]+ (10.7).
General procedure for the synthesis of nitriles 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluorobutanoic acid 2a, b by C-alkylation of indole derivatives 4a, b with alkene 1
The dichloromethane (3.0 ml), indole derivatives 4a, b (1.78 mmol) and alkene 1 (0.26 g, 1.78 mmol) in 1 ml DCM were added sequentially into a round-bottom flask with magnetic stir bar under the argon atmosphere at 0-5°C. The reaction mixture was stirred for 4 hours. When the reaction done, solution was diluted with dichloromethane (20 ml) and filtered through a 1 cm layer of silica gel and the solvent was removed under reduced pressure to give the desired product.
2-[2,2,2-Trifluoro-1-(1H-indole-3-yl)-ethyl]malononitrile (2a)
Obtained: 0.42 g, yield 92 %, light yellow rosin-shaped oil, Rf = 0.5 (PE+EA, 0.8+0.2).
1Н NMR (CDCl3, δ, ppm., J/Hz): 4.37-4.45 (2Н, m, overlap of two signals -CH-); 7.24-7.64 (5H, m, Ar); 8.54 (1Н, br. s., NH).
13С NMR DEPT 135 (CDCl3, δ, ppm., J/Hz): 25.29 (s,-CH(CN)2); 42.40 (q, 30.5 Hz, CF3-СН-); 102.54; 110.71 (s, -CN); 110.81 (s, -CN); 112.06; 117.73; 121.08; 123.44; 124.47 (q, 280.9 Hz, -CF3); 124.72; 126.13; 135.60.
19F NMR (CDCl3, δ, ppm.): -67.89 (s, -CF3).
MS (EI, 70 eV), m/z (Irel (%)): 262.9 [М]+ (9.4); 198.2 [M-СН(СN)2]+ (100).
HRMS (ESI, neg. ions), C13H8F3N3, found m/z: 262.0595 [M-H]-. Calculated: 262.0598.
2-[2,2,2-Trifluoro-1-(2-methyl-1H-indole-3-yl)ethyl]malononitrile (2b)
Obtained: 0.44 g, 89% yield, light beige powder, Rf = 0.2 (PE+EA, 0.8+0.2).
1Н NMR (CDCl3, δ, ppm., J/Hz): 2.32 (3Н, s, -Ме); 4.21-4.30 (1Н, m, CF3-СН-); 4.92 (1Н, d, 9.6 Hz, -CH(CN)2); 6.95-7.45 (4Н, m, Ar); 10.37 (1Н, br. s., NH).
13С NMR DEPT 135 (CDCl3, δ, ppm., J/Hz): 11.46 (s, -Ме); 23.58 (s, -CH(CN)2); 43.37 (q, 30.7 Hz, CF3-СН-); 97.98; 110.92 (s, -CN); 111.16; 111.27 (s, -CN); 118.02; 119.85; 121.30; 124.72 (q, 281.7 Hz, -CF3); 125.15; 135.45; 136.98.
19F NMR (CDCl3, δ, ppm.): -65.64 (s, -CF3).
MS (EI, 70 eV), m/z (Irel (%)):277 [М]+ (6.6); 212.1 [M-СН(СN)2]+ (100). HRMS (ESI, neg. ions), C14H10F3N3, found m/z: 276.0759 [M-H]-. Calculated: 276.0754.
Three-component one-pot synthesis of 2-[2,2,2-trifluoro-1-(1-methyl-1H-indole-3-yl) ethyl]malononitrile (2c)
The DCM (3.0 ml), malononitrile (0.132 g, 2.0 mmol), trifluoroacetaldehyde ethyl hemiacetal (0.288 g, 2.0 mmol), triethylamine (0.1 g) and molecular sieves 4A were added sequentially into a round-bottom flask with magnetic stir bar under the argon atmosphere. The reaction mixture was stirred at room temperature for four hours. Then, with a syringe add N-methylindole 4c (0.262 g, 2 mmol) dissolved in 1 ml of DCM. After a day, the reaction mixture was diluted (20 ml) with a mixture of DCM and PE (1/1) and transferred to a chromatographic column containing 100 ml of silica gel. The reaction mass is eluted with a mixture of DCM with PE (1/1), gradually increasing the solvent gradient by adding EA to 3%. The solution of the purified product is evaporated at reduced pressure and then re-evaporated with 20 ml of DCM. Received: 0.41 g, 74% yield, light yellow rosin-shaped oil, Rf = 0.4 (PE+EA, 0.8+0.2).
1Н NMR (CDCl3, δ, ppm., J/Hz): 3.82 (3H, s, -Ме); 4.36-4.44 (2Н, m, overlap of two signals -СН-); 7.25-7.63 (4Н, m, Ind); 7.44 (1Н, br. s., Н(2) Ind).
13С NMR DEPT 135 (CDCl3, δ, ppm., J/Hz): 25.44 (s, -CH(CN)2); 33,28 (s, N-Me); 42.53 (q, 30.4 Hz, CF3-СН-); 100.90; 110.19; 110.65 (s, -CN); 110.72 (s, -CN); 117.81; 120.82; 123.08; 124.49 (q, 281.4 Hz, -CF3); 126.92; 128.82; 136.61.
19F NMR (CDCl3, δ, ppm.): -67.98 (s, -CF3).
MS (EI, 70 eV), m/z (Irel (%)): 277 [М]+ (14.3); 212 [M-СН(СN)2]+ (100).
HRMS (ESI, neg. ions), C14H10F3N3, found m/z: 276.0751 [M-H]-. Calculated: 276.0754.
General procedure for producing of nitriles 3-(1H-indole-3-yl)-2-cyano-4,4,4-trifluoro-butanoic acid 2c - f by three-component one-step synthesis
The dichloromethane (3.0 ml), malononitrile (0.264 g, 4.0 mmol), indole derivatives 4c - f (4.0 mmol), trifluoroacetaldehyde ethyl hemiacetal (0.58 g, 4.0 mmol), triethylamine (0.15 g) and molecular sieves 4A were added sequentially into a round-bottom flask with magnetic stir bar under the argon atmosphere. The reaction mixture was stirred at room temperature for overnight. After completion of the reaction, the solvent was removed under reduced pressure, and the crude residue was purified by silica gel column chromatography with PE/DCM as eluent, gradually increasing the solvent gradient by adding ethyl acetate to 3%. The solution was evaporated at low pressure and then re-evaporated with 20 ml of DCM to give the desired product.
2-[2,2,2-Trifluoro-1-(1-methyl-1H-indole-3-yl)ethyl]malononitrile (2c)
Obtained: 0.9 g, 81% yield, light yellow rosin-shaped oil, Rf = 0.4 (PE+EA, 0.8+0.2).
The spectral data are given earlier.
2-[1-(1-Benzyl-1H-indole-3-yl)-2,2,2-trifluoroethyl]malononitrile (2d)
Obtained: 1.09 g, 78% yield, light yellow rosin-shaped oil, Rf = 0.4 (PE+EА, 0.8+0.2).
1Н NMR (CDCl3, δ, ppm., J/Hz): 4.40-4.42 (2Н, m, overlap of two signals -СН-); 5.39 (2Н, s, -СН2-); 7.11-7.64 (9Н, m, Bnz, Ind,); 7.50 (1Н, s, Н(2) Ind).
13С NMR DEPT 135 (CDCl3, δ, ppm., J/Hz): 25.28 (s, -CH(CN)2); 42.49 (q, 30.4 Hz, CF3-СН-); 50.46 (s, -СН2-); 101.70; 110.62 (s, -CN); 110.75; 110.80 (s, -CN); 118.04; 121.01; 123.23; 124.46 (q, 280.9 Hz, -CF3); 126.73; 127.05; 127.98; 128.49; 128.97; 136.16; 136.40.
19F NMR (CDCl3, δ, ppm.): -67.85 (s, -CF3).
MS (EI, 70 eV), m/z (Irel (%)): 353 [М]+ (8.9); 288 [M-СН(СN)2]+ (50.9); 91 [C7H7]+ (100).
HRMS (ESI, neg. ions), C20H14F3N3, found m/z: 352.1076 [M-H]-. Calculated: 352.1067.
2-[1-(5-Bromo-1H-indole-3-yl)-2,2,2-trifluoroethyl]malononitrile (2e)
Obtained: 0.77 g, yield 56 %, light yellow rosin-shaped oil, Rf = 0.2 (PE+EА, 0.8+0.2).
1Н NMR (CDCl3, δ, ppm., J/Hz): 4.28-4.35 (1Н, m, CF3-СН-); 4.42 (1Н, d, 5.2 Hz, -CH(CN)2); 7.23 (1Н, d, 6.2 Hz, Н(7)); 7.33 (1Н, br. d., 8.6 Hz, Н(6)); 7.50 (1Н, br. s., Н(4)); 7.72 (1Н, s, Н(2)); 8.77 (1Н, br. s., NH).
13С NMR DEPT 135 (CDCl3, δ, ppm., J/Hz): 25.33 (s, -CH(CN)2); 42.51 (q, 30.7 Hz, CF3-СН-); 102.33; 110.49 (s, -CN); 110.57 (s, -CN); 113.58; 114.53; 120.40; 124.27 (q, 281.0 Hz, -CF3); 125.86; 126.58; 127.95; 134.39.
19F NMR (CDCl3, δ, ppm.): -67.94 (s, -CF3).
MS (EI, 70 eV), m/z (Irel (%)): 341 [M]+ (11.6); 276 [M-СН(СN)2]+ (100).
HRMS (ESI, neg. ions), C13H7BrF3N3, found m/z: 339.9695 [M-H]-. Calculated: 339.9703.
2-[2,2,2-Trifluoro-1-(2-phenyl-1H-indole-3-yl)ethyl]malononitrile (2f).
Obtained: 1.02 g, 75% yield, light beige powder, Rf = 0.3 (PE+EА, 0.8+0.2).
1Н NMR (CDCl3, δ, ppm., J/Hz): 4.34-4.43 (1Н, m, CF3-СН-); 4.67 (1Н, d, 10.4 Hz, -CH(CN)2); 7.26-7.69 (9Н, m, Ind+Ph); 8.56 (1H, br. s., NH).
13С NMR DEPT 135 (CDCl3, δ, ppm., J/Hz): 23.46 (s, -CH(CN)2); 43.64 (q, 30.5 Hz, CF3-СН-); 98.61; 110.69 (s, -CN); 110.99 (s, -CN); 111.99; 119.04; 120.30; 122.29; 124.51; 124.77 (q, 282.4 Hz, -CF3); 128.72; 128.88; 129.24; 130.76; 136.20; 140.93.
19F NMR (CDCl3, δ, ppm.): -65.03 (s, -CF3).
MS (EI, 70 eV), m/z (Irel (%)): 339 [M]+ (9.7); 274 [M-СН(СN)2]+ (100).
HRMS (ESI, neg. ions), C19H12F3N3, found m/z: 338.0921 [M-H]-. Calculated: 338.0911.
2-(2,2,2-Trifluoro-1-hydroxyethyl)malononitrile (3).
The substance is not stable during storage and heating and was not purified individually. The CS of 1H, 19F NMR and the mass spectrum are given for the reaction mass of equimolar amounts of TFAE (7.2 g, 0.05 mol), malononitrile (3.3 g, 0.05 mol) and triethylamine (0.35 g) in DCM (5 ml) after reaction done. The CS of protons, multiplicity, and J-coupling depend on the composition of the mixture.
1H NMR (no solvent, δ, ppm., J/Hz): 4.73 (1H, d, 2.3 Hz, -СН(СN)2); 4.78-4.85 (1H, m, СF3-СH-).
19F NMR (CDCl3, δ, ppm.): -77.25 (s, -CF3).
MS (EI, 70 eV), m/z (Irel (%)): 164 [М]+ (2.1); 146 [М-H2O]+ (9.3).
Acknowledgments
This work was performed with the financial support from Ministry of Science and Higher Education of the Russian Federation. The contribution of Center for molecular composition studies of A.N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences (Moscow, Russia) is gratefully acknowledged. High resolution mass spectrometry was performed in the Department of Structural Studies of N.D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences (Moscow, Russia).
References
- A. V. Fokin, V. Yu.Tyutin, N. D. Chkanikov, The chemistry of perfluoroalkyl-substituted dicyanoethylenes, Russ. Chem. Rev., 1992, 61, 766
- N. D. Chkanikov, K. V. Komarov, V. Yu. Tyutin, A. F. Kolomiets, A. V. Fokin, C-Alkylation of indoles with 1,1-bis(trifluoromethyl)-2,2-dicyanoethylene and 2-trifluoromethyl-3,3-dicyano-acrylic acid esters, Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1991, 5, 1193-1195.
- N. D. Chkanikov, A. S. Golubev, E. V. Belyaeva, New Approaches to the Synthesis of CF2X- Substituted Heterocyclic Anttitumor Cytostatic Agents, INEOS OPEN, 2019, 2(2), 33-40. DOI: IO.32931/io1906r.
- V. Yu. Tyutin, N. D. Chkanikov, A. F. Kolomietz, A. V. Fokin, Synthessis of esters of 3,3-dicyano-2-(trifluoromethyl)acrylic acid and their reactions with arylamines, J. Fluorine Chem., 1991, 51, 323-334.
- W. J. Middleton, 1,1-Dicyano-2,2-bis(trifluoromethyl)ethylene, J. Org. Chem., 1965, 30, 1402-1407.
- A. S. Golubev, P. V. Pasternak, A. F. Shidlovskii, L. N. Savel`eva, B. B. Averkiev, V. N. Nesterov, M. Yu. Antipin, A. S. Peregudov, N. D. Chkanikov., Synthesis and some heterocyclisation reactions of CF2H-and CF2Cl-substituted 1,1-dicyanoethylenes, J. Fluorine Chem., 2002, 114, 63-74.
- O. Yu. Fedorovskii, A. Yu. Volkonskii, A. S. Golubev, Yu. Ya. Spiridonov, N. D. Chkanikov, Synthesis of ethyl α-nitro-β-trifluoromethyl acrylate and β-trifluoromethyl-substituted tryptophan analogs and their plant growth regulating activity, Russian Chemical Bulletin, 2017, 66(6), 1116-1121.
- R. J. Sundberg, Indoles; San Diego, Academic. San Diego, 1996.
- Y. Maki, H. Kimoto, S. Fujii, M. Senga, L. A. Cohen., Thermal condensation of indoles with trifluoroacetaldehyde, Journal of Fluorine Chemistry, 1988, 39(1), 47-59.
- Y. Gong, K. Kato Recent., Applications of Trifluoroacetaldehyde Ethyl Hemiacetal for the Synthesis of Trifluoromethylated Compounds, Current Organic Chemistry 2004, 8(17), 1659-1675. DOI: 10.2174/1385272043369683.
- S. Peerannawar, A. Sood, A. Brown, C. Schäfer, J. Alonzo, S. Sutton, M. Christianson, R. Stocking, N. Naclerio, B. Török, S. M. Landge., Effect of solvent polarity on the regioselective hydroxyalkylation of indole with trifluoroacetaldehyde hemiacetals., Structural Chemistry, 2019, 30, 1941-1956.
- Yan-Hong He, Jian-Fei Cao, Rui Li, Yang Xiang, Da-Cheng Yang, Zhi Guan, L-Proline-catalized multicomponent synthesis of 3-indole derivatives, Tetrahedron, 2015, 71, 9299-9306.
- Zhu J. P., Bienayme` H., Multicomponent Reactions; Wiley-VCH: Weinheim, 2005.
- Trost B. M., Atom Economy. A Challenge for Organic Synthesis. Angew. Chem. Int. Ed. Engl., 1995, 34(3), 259–281.
- Tsedilin A. M., Fakhrutdinov A. N., Eremin D. B., Zalesskiy S. S., Chizhov A. O., Kolotyrkina N. G., Ananikov V. P., How sensitive and accurate are routine NMR and MS measurements?, Mendeleev Comm., 2015, 25, 454.
ARTICLE INFO
Received 08 February 2021
Accepted 15 February 2021
Available online February 2021
Recommended for publication by PhD M. A. Manaenkova
Fluorine Notes, 2021, 134, 3-4
