Received: June 2021
DOI 10.17677/fn20714807.2021.04.01
Fluorine Notes, 2021, 137, 1-2
DIRECTED SYNTHESIS OF COPOLYMERS BASED ON FLUORINE-CONTAINING (METH)ACRYLATE DERIVATIVES
Chekurov K.E., Barabanova A.I.
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow, Russia
e-mail: chekurov@polly.phys.msu.ru
Abstract: The review considers and analyzes the literature data on the directed synthesis of (co)polymers based on fluorine-containing acrylate and methacrylate derivatives, published over the past two decades.
Keywords: RAFT-polymerization, copolymers, fluoroacrylates, fluoromethacrylates
Introduction
Interest in the directed synthesis of fluorine-containing copolymers is due to their unique properties, such as high thermal and chemical resistance to environmental influences and aging, low values of free surface energy, dielectric constant, refractive index, etc. [1]–[3]. To obtain fluorine-containing copolymers, fluorinated derivatives of (meth)acrylate are most often used because of their high reactivity in polymerization reactions, as well as the convenience and low cost of their synthesis.
Recently, directed synthesis of fluorinated (co)polymers with the required properties has been carried out by methods of reversible-deactivation radical polymerization (RDRP), such as atom transfer radical polymerization (ATRP), radical polymerization under conditions of reversible inhibition in the presence of nitroxides (nitroxide-mediated polymerization, NMP) and radical addition-fragmentation transfer (RAFT) polymerization [4]–[6]. Of the existing RDRP methods, RAFT polymerization is the simplest and most versatile method for controlled polymer synthesis, since it proceeds under relatively mild conditions and is suitable for a wide range of monomers.
In this review, we will consider in detail the work on RAFT polymerization of fluorinated (meth)acrylates.
Synthesis of fluorine-containing copolymers by RAFT polymerization
In 1998, the first reports of the Rizzardo group appeared about the use chain transmitters for directed synthesis of polymers – sulfur-containing compounds of general formula Z-C(=S)-S-R, added to the reaction mixture in catalytic amounts. In the English-language literature, this process is called “reversible addition-fragmentation chain transfer (RAFT–polymerization)” and the chain transmitters are called “chain transfer agent (CTA)” or “RAFT-agent” [7]. In the Russian-language literature the terms “pseudo-living radical polymerization with reversible chain transfer by addition-fragmentation mechanism (RAFT–polymerization)” and “RAFT-agent” are used respectively.
The mechanism of RAFT-polymerization differs from classical radical polymerization by the presence (along with elementary reactions of initiation, growth, termination and chain transfer) stages of the addition of the growing radical to the CTA, followed by fragmentation (decomposition) of the intermediate (Int1 and Int2) and formation of the macroCTA, which further takes part in the process of reversible chain transfer, and growth radical (R∙ и Pm), which re-initiates polymerization. The scheme of the process is presented below.
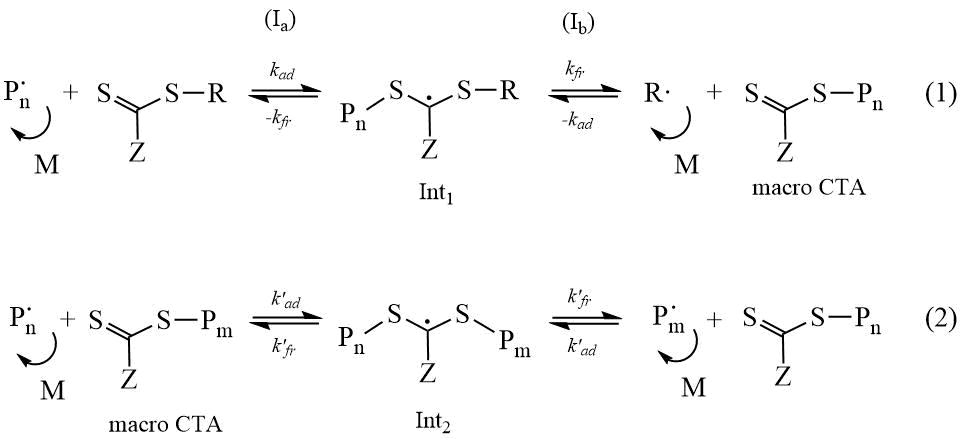
Scheme 1. Mechanism of RAFT polymerization.
Polymerization control is provided by adding CTA into the system. As a rule, these compounds are classified as dithio- or trithio compounds (Scheme 2). Stabilizing Z- and leaving R-groups have a significant effect on the direction of the polymerization reaction (toward free radical polymerization or toward fragmentation of the intermediate) and on the kinetics of the process. It is important to correctly select the corresponding groups depending on the nature of the monomer used. Below are presented CTAs used in the polymerization of fluorinated (meth)acrylates (Scheme 2).
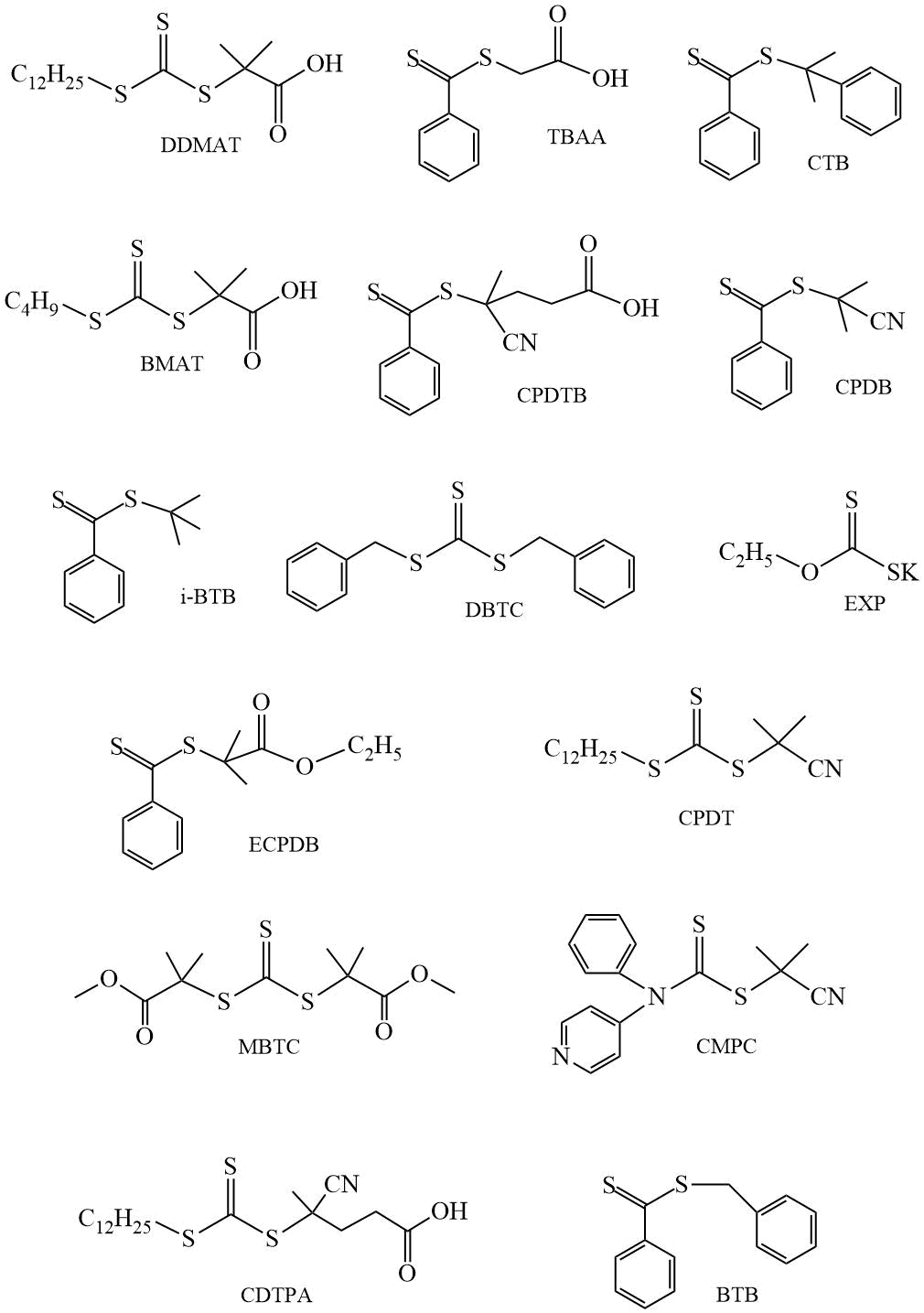
Scheme 2. Examples of CTAs.
RAFT polymerization of fluorine-contaning acrylates
Works devoted to RAFT polymerization of fluorinated acrylates (Scheme 3) appeared only 6 years later, after the discovery of RAFT polymerization in the presence of thio compounds, and the number of these works is still small [8]-[19].
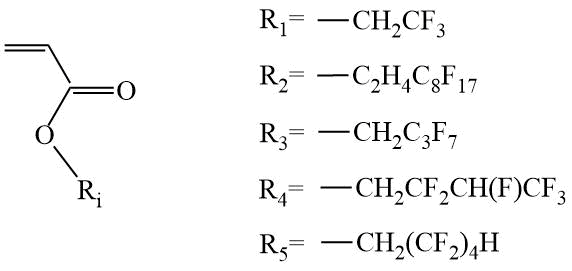
Scheme 3. Examples of fluorine-containing acrylates.
In 2004, Ma et al. performed a detailed study of the RAFT polymerization of perfluorooctylethyl acrylate (PFOEA) in the presence of two CTAs based on polyethylene oxide (PEO) with end-capped groups from 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid (DDMAT) (PEO-CTA1) and from thiobenzoylthio acetic acid (TBAA) (PEO-CTA2) (Scheme 4) [8]. Polymerization was carried out in trifluorotoluene (TFT) at 65ºС with azobisisobutyronitrile (AIBN) as an initiator at molar ratio of [PFOEA] / [CTA] / [AIBN] = 19.32 mmol / 0.537 mmol / 0.108 mmol. In the presence of both CTAs, the reaction proceeded to high conversions (70 and 97.8 % for PEO-CTA1 and PEO-CTA2 respectively) without the formation of by-product by uncontrolled radical polymerization of PFOEA. The authors believe that the polymerization has “living” character, since the experimental values of Mn calculated from the composition of synthesized polymers do not exceed 25 kDa and are in good agreement with the theoretical values.
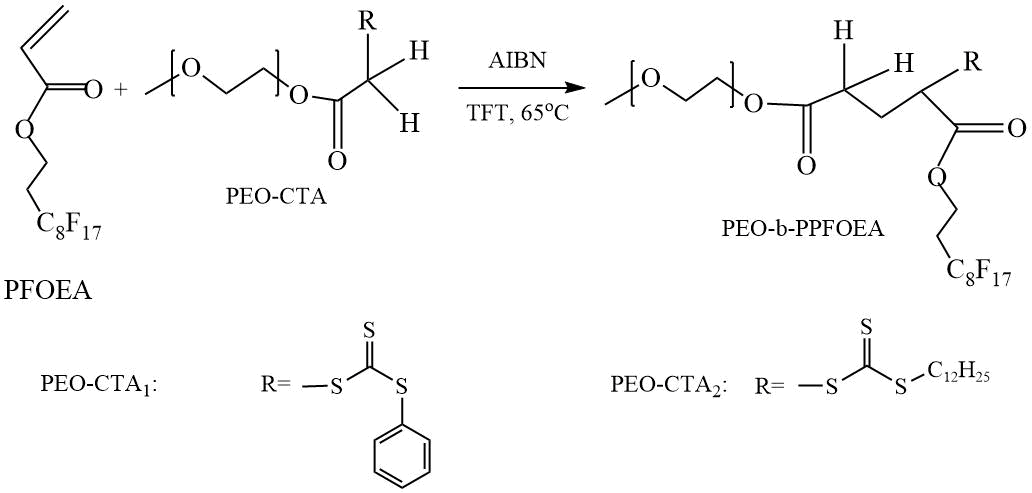
Scheme 4. Synthesis of DC (PEO-PPFOEA) [8].
Grignand et al. synthesized amphiphilic random copolymers and diblock copolymers (DC) of PFOEA and 2-hydroxyethyl acrylate (HEA) [9]. The two-stage DC synthesis began with the polymerization of PFOEA in TFT in presence of DDMAT. Copolymers were prepared in a mixed solvent TFT:DMF (1:1 vol.). The study of the kinetics of polymerization by NMR spectroscopy showed that Mn values linearly increase with conversion, which, in the authors’ opinion, indicates a controlled character of polymerization.
Skrabania et al. [10] reported the preparation of linear amphiphilic triblock copolymers (TC). The authors used a PEO-CTA with a butyl trithiocarbonate end-capped group as the CTA. At the first stage of the synthesis, DC was obtained by polymerizing butyl acrylate (BA) or 2-ethylhexyl acrylate (EHA) in the presence of PEO-CTA, and at the second stage, by polymerizing a fluorine-containing PFOEA in the presence of a diblock copolymer CTA, TC was obtained (Scheme 5).
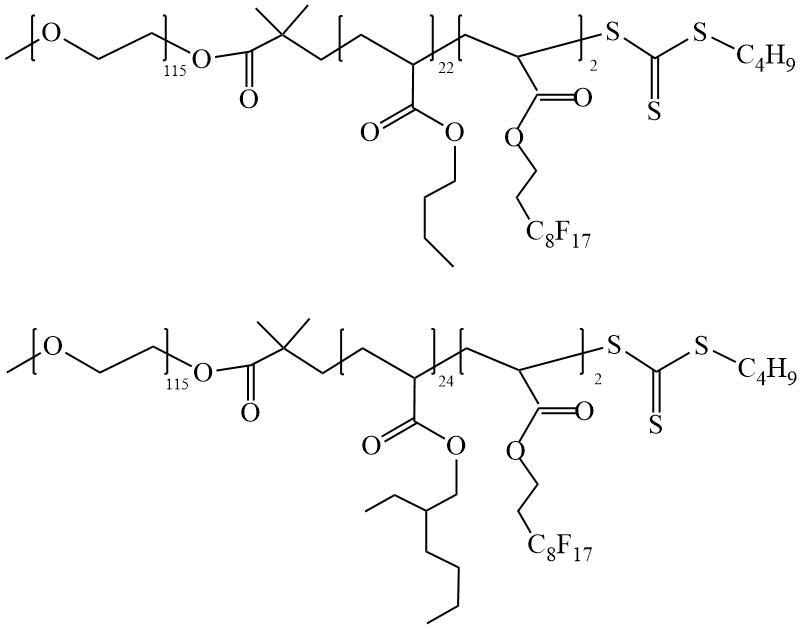
Scheme 5. Synthesis of TC based on PFOEA [10].
In [11] amphiphilic DCs of 2,2,2,-trifluoroethyl acrylate (TFEA) were synthesized by RAFT polymerization of TFEA in the presence of PEO-CTA with a dithiobenzoate end-capped group. The obtained DCs were used as filler for epoxy resin to improve its surface properties. The values of contact angles (CA) for wetting with water (WCA) and ethylene glycol of coatings made of composite thermosets containing 40 wt.% DC dispersed in the epoxy matrix were: θН2О = 101.2º and θC2H6О2 = 84.5º, respectively, compared with θН2О = 74.3º and θC2H6О2 = 60.7º for the “unfilled” epoxy network. The adding of DCs led not only to an increase in CA, but also decrease in the free surface energy from 29.4 mJ/m2 (for epoxy resin) to 14.9 mJ/m2 (for a composite). The authors attributed the observed improvement in surface properties to the presence of fluorine atoms ([F] = 6.3 wt. % according to EDS data) on the surface of films made of composite thermosets, which is probably due to the microphase separation of DC in the epoxy matrix. In their other work [12], the authors also achieved an improvement in the surface properties of epoxy network (θН2О = 102º and θC2H6О2 = 83º) and a decrease in the energy characteristics of its surface up to 16.4 mJ/m2 due to the adding of DC into epoxy resin obtained by polymerizing TFEA with another comonomer – glycidyl methacrylate (GMA) by using cumyl dithiobenzoate (CTB) as a CTA (Scheme 6).
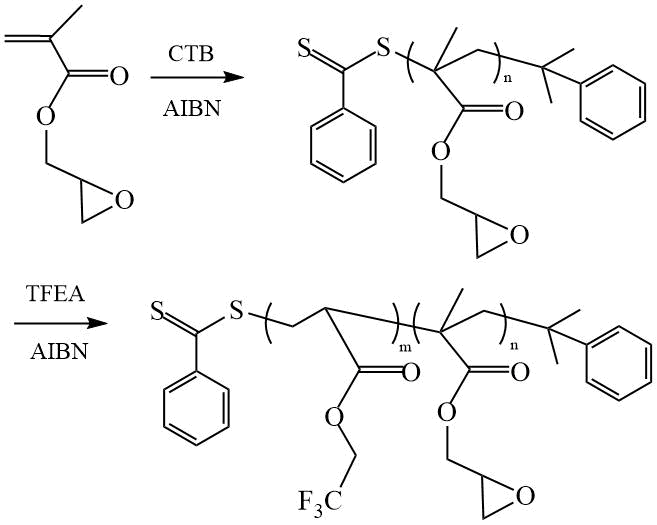
Scheme 6. Synthesis of DC (PGMA-PTFEA) [12].
Li et al. synthesized amphiphilic TC, consisting of polyethylene glycol (PEG) and copolymer block, by copolymerization of methyl acrylate (MA) and 4-azophenylmeth acrylate (APMA), or 2,2,3,4,4,4-hexafluorobutyl acrylate (HFBA) in the presence of PEG-CTA with a terminal dodecyltrithiocarbonate group [13] (Scheme 7).
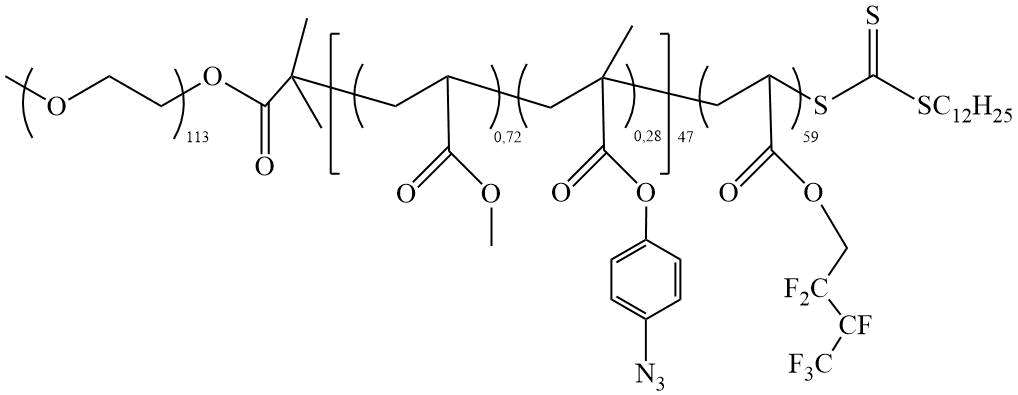
Scheme 7. TC (PEG-(MA-co-APMA)-HFBA) [13].
The application of the obtained TC on the hydrophilic cotton fabric transform it into a superhydrophobic material with θН2О =155º (> 150°) and the value of the CA hysteresis (CAH) = 2º (CAH < 10°).
In a series of works, Koiry et al. [14]–[16] reported a detailed study of RAFT polymerization of 2,2,3,4,4,4-heptafluorobutyl acrylate (HFBA). In [14], the synthesis of butyl acrylate (BA) and HFBA copolymers was carried out in the presence 4-cyano-4-((thiobenzoyl)-sulfanyl) pentanoic acid (CPDTB) in dioxane at 90ºС. The number average molecular weight of the obtained narrowly dispersed copolymers (Mw/Mn = 1.21 ÷ 1.26), depending on the composition, varies in the range of Мn = 16 ÷ 21 kDa. In [15], the authors reported on the copolymerization of HFBA with another comonomer methyl methacrylate (MMA) in the presence of 2-cyano-2-propyl dithiobenzoate (CPDB) (Scheme 8).
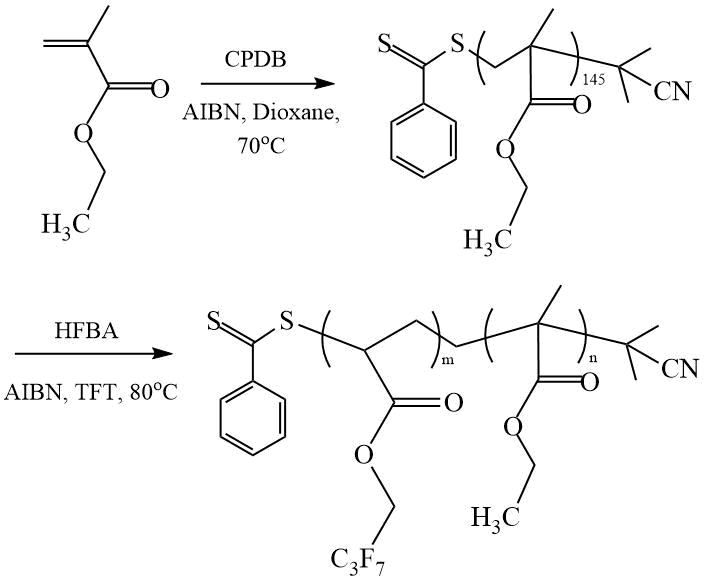
Scheme 8. Synthesis of DC (MMA-HFBA) [15].
The ability of the obtained MMA-HFBA copolymers to self-assembly was studied in mixtures of tetrahydrofuran and methyl ethyl ketone (THF-MEK) of various compositions. It is shown that in a mixed solvent THF:MEK = 3:2 copolymers are collected in tubes ( 250 nm). The authors believe that the ability of these DCs to form tubes under the influence of external factors can be used to create self-healing coatings. In [16], amphiphilic DCs of poly(ethylene glycol) methyl ether methacrylate (PEGMA) and HFBA were also synthesized in the presence of CPDB in dioxane at 85ºС.
The work [17] describes the preparation of DC by RAFT polymerization of 2‑(dimethylamino)ethyl methacrylate DMAEMA and HFBA using CTB as a low molecular weight CTA. Absorbents based on a highly porous material from synthesized DC perfectly absorb oil from aqueous media, while maintaining high values of θН2О = 140º, even after 3 hours of annealing at 200ºС.
The study of the polymerization of PFOEA in the presence of styrene (St) was carried out by Dai et al. [18]. DC synthesis was performed by two-stage RAFT polymerization. At the first stage, polystyrene (PSt) was obtained in the presence of dibenzyl carbonotrithioate (DBTC) in bulk, and at the second stage, DC was synthesized in a mixture of solvents THF:TFT = 1:1 by volume (Scheme 9).
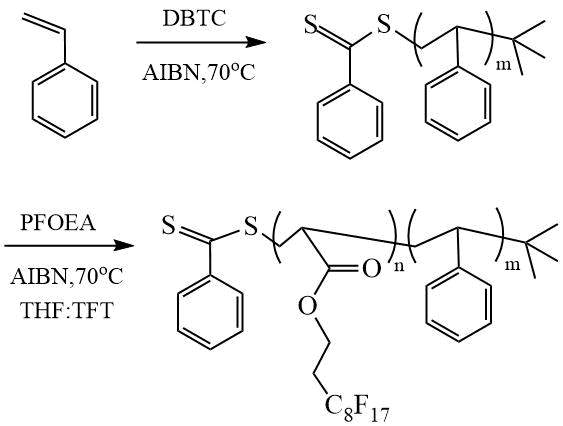
Scheme 9. Synthesis of DC (PSt-PPFOEA) [18].
In a recently published paper by Grigoreva et al. [19] considers the RAFT polymerization of 2,2,3,3,4,4,5,5-octofluoropentyl acrylate (OFPA) in the presence of a low molecular CTA DBTC in benzene at 80ºС, as well as the copolymerization of OFPA with acrylic acid (AA) and tret-butyl acrylate. The authors were the first to quantitative characterized the efficiency of DBTC in the polymerization of fluoroacrylate. The criterion for the effectiveness of the CTA is the value of constant of chain transfer to the CTA Сtr. It is conventionally assumed that effective CTA are those for which С >> 1. In [19], the Сtr value was determined using the approach described in [20], and it was Сtr 14 (>> 1). Under the found conditions, block copolymers with Мn = 6.9 ÷ 95 kDa and Mw/Mn = 1.18 ÷ 2.85 and random copolymers with Мn = 0.9 ÷ 63.2 kDa and Mw/Mn = 1.18 ÷ 5.76 were obtained. The study of the aggregation behavior of copolymers at the air/water interface by the Langmuir monolayer technique showed that a change in the acidity of the subphase can affect the type of self-organization of macromolecules in the monolayer due to the ionization of carboxyl groups in AA. Under alkaline conditions, the size of micelles increases in comparison with neutral ones and is 90-100 nm.
RAFT polymerization of fluorine-contaning methacrylates
Along with fluorinated acrylates, methacrylates (Scheme 10) are also widely used in the directed synthesis of fluorine-containing block copolymers by RAFT polymerization [21]-[33].
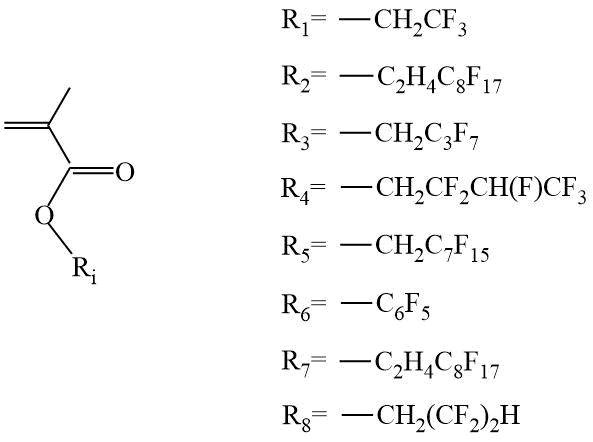
Scheme 10. Examples of methacrylate monomers.
In [21], Eberhardt et al. carried out the RAFT polymerization of pentafluorophenyl methacrylate (PFPMA) in the presence of two CTA: CPDTB and CTB. The reaction was carried out in dioxane in the presence of AIBN as initiator. The obtained homopolymers were characterized by low values of the polydispersity index (Mw/Mn < 1.3 for CTB and <1.15 for CPDTB). Subsequently, amphiphilic DC were obtained by polymerization of MMA, N-acryloylmorpholine (N-AM), and N,N-diethyl acrylamide (DEAA). DC obtained by copolymerization in the presence of CPDTB are characterized by lower polydispersity (Mw/Mn <1.30, for DC with Mn=50 kDa), than DC obtained in the presence of CTB (Mw/Mn <1.40) for DC with lower Mn = 20 ÷ 40 kDa.
Gradient, statistical and DC of trifluoroethyl methacrylate (TFEMA) and 2‑methacryloyloxyethylphosphorylcholine (MPC) were synthesized by RAFT polymerization with CPDTB as a CTA [22]. The presence of hydrophilic and biocompatible MPC units that mimic phospholipids makes these DCs promising for use in biomedicine.
Another article devoted to the study of the surface properties of coatings based on fluorine-containing amphiphilic TC was carried out by Guan et al. [23]. The authors synthesized copolymers based on polydimethylsiloxane, 2,2,3,3,4,4,4-heptafluorobutyl methacrylate and polystyrene (PDMS-HFBMA-PSt). The reaction proceeded in toluene at 60ºС, and potassium O-ethylxanthate (EXP) (RAFT/MADIX process) was used as CTA. Films were cast from solutions of the obtained copolymers in THF onto glass substrates. It is shown that, as a result of self-organization, the repellent properties of coatings based on TCs were superior to those for DCs. For example, the WCA for a TC film (θН2О =122º) is 15° higher than that for a DC film (θН2О =107º). The value of the surface energy for the TC film (10,64 mJ/m2),on contrary, is lower than for the DC film (18,72 mJ/m2).
Mya et al. investigated the RAFT polymerization of hexafluorobutyl methacrylate (HFBMA) and obtained DC HFBMA and poly(propylene glycol) acrylate (PGA) in the presence of CPDB in TFT [24] (Scheme 11).
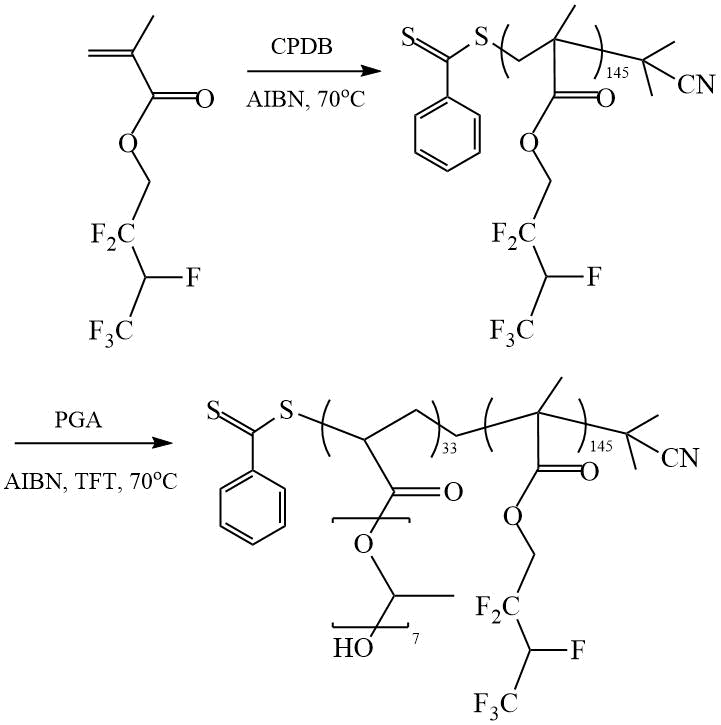
Scheme 11. Synthesis of DC (HFBMA-PGA) [24].
Liu et al. [25] hydrophobized ramie fibers by polymerization of TFEMA in supercritical СО2 in the presence of 2-(ethoxycarbonyl)prop-2-yl dithiobenzoate (ECPDB) grafted onto fibers (Scheme 12). After receiving the modified fiber, it is loaded into a СО2 reactor with a monomer, an initiator, and portion of the “free” CTA. The WCA values of the modified fibers θН2О reached 149º.
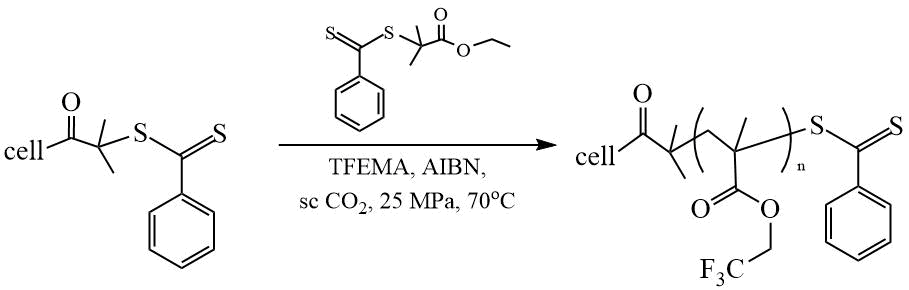
Scheme 12. Synthesis of graft copolymer based on TFEMA [25].
Li et al. carried out the synthesis of fluorine-containing DC based on MMA and TFEMA using CTB as CTA [26] (Scheme 13). Films cast from DC solutions in THF onto glass are characterized by increased WCA and CA of ethylene glycol θН2О = 113º, а θC2H6О2 = 82,4º.
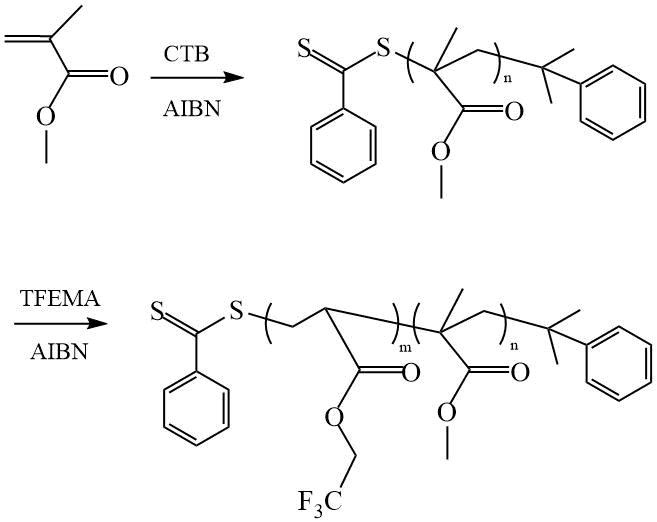
Scheme 13. Synthesis of DC PMMA-PTFEMA [26].
Huo et al. [27] synthesized amphiphilic TC by polymerizing 2-diethylaminoethyl methacrylate (DEAEMA), benzyl methacrylate (BzMA), and perfluorooctylethyl methacrylate (PFOEMA) in the presence of CPDTB as CTA (Scheme 14), and studied the morphology of multicomponent micelles (MCM) from obtained TC in ethanol solution.
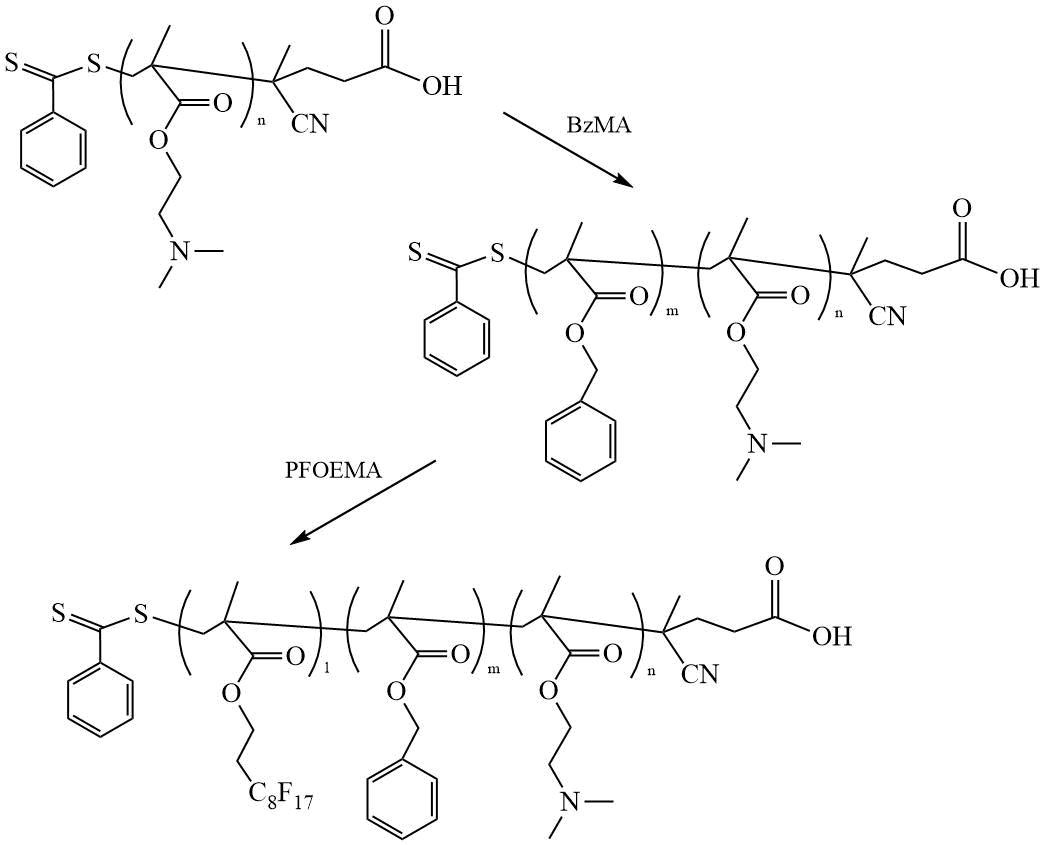
Scheme 14. Synthesis of TC (DEAEMA-BzMA-PFOEMA) [27].
The study of the morphology of MCM from TC by TEM showed that with an increase in the mass of the fluorinated block, vesicles from TC are converted first into spheres and then into cylinders. Another work by the same authors [28] reported the preparation of TC by polymerization of DMAEMA with BzMA and perfluorohexylethyl methacrylate (PFHEMA) in the presence of CPDTB.
Not so long ago, Chakrabarti et al. obtained superhydrophobic coatings on glass with θН2О up to 151º from copolymers of 4-vinyl pyridine and vinyl triethoxysilane with TFEMA obtained by polymerization in the presence of DDMAT [29] (Scheme 15).
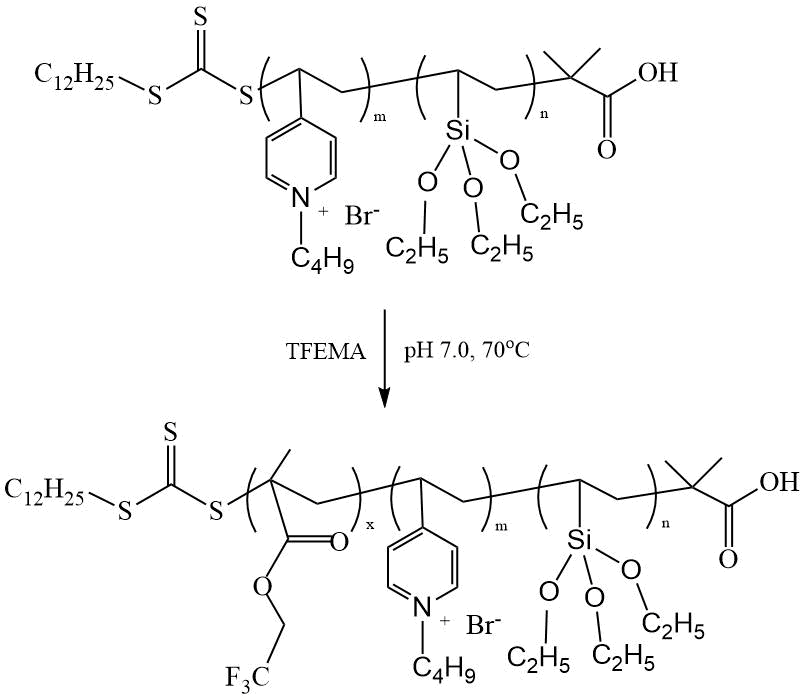
Scheme 15. Polymerization of TFEMA in the presence of macroCTA [29].
Wang et al. [30] reported on the preparation of DC based on perfluoroheptylmethyl methacrylate (PFHMMA) and hydroxystyrene (HSt) and on the creation of thin films from the synthesized DCs. Polymerization was carried out in the presence CPDB in hexafluoroisopropanol (HFIP), followed by the removalof the protective tetrahydropyran groups, which prevent side reactions with the participation of hydroxyl groups during polymerization (Scheme 16). The obtained copolymers with a volume fraction of fPHSt = 0.4 ÷ 0.69, had Mn = 2.2 ÷ 9.5 kDa and polydispersity Mw/Mn = 1.08 ÷ 1.12. The morphology of the films was studied by SAXS and TEM. It is shown that lamellar morphology is characteristic of all DC films. The minimum value of the period of the lamellar structure was 9.8 nm. However, during a short annealing at a moderate temperature (80ºС, 1 min), the lamellar period decreases to values less than 5 nm.
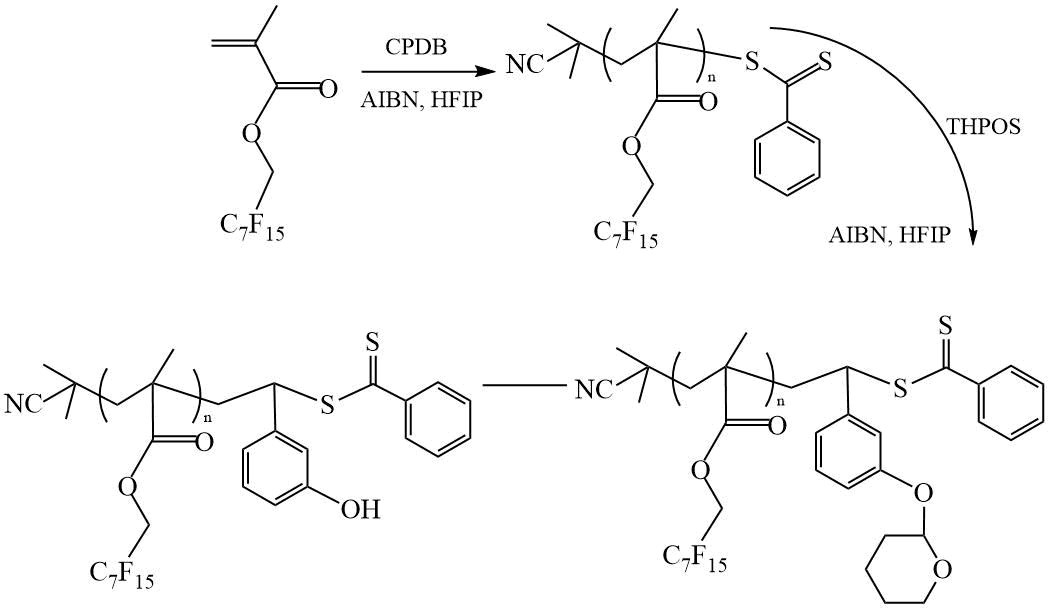
Scheme 16. Synthesis of DC PFHMMA-PHSt [30].
The work [31] is devoted to the preparation of DC based on poly[poly(ethylene glycol) methyl ether methacrylate] (PEGMA) and poly(2,3,4,5,6-pentafluorobenzyl methacrylate) (PFBMA) with the subsequent substitution of the fluorine atom in the para-position with various thiols (Scheme 17). CPDTB was used as CTA; reaction was carried out in ethanol at 70ºС. It was found that DC solutions spontaneously combine to form various nanoparticles, the morphology of which depends on the nature of the substituting thiol and the molecular weight of the copolymer. It was found that the solvophobicity of the nanoparticle core is a more important factor determining morphological transitions compared to the molecular weight of the polymer block that makes up the nanoparticle core.
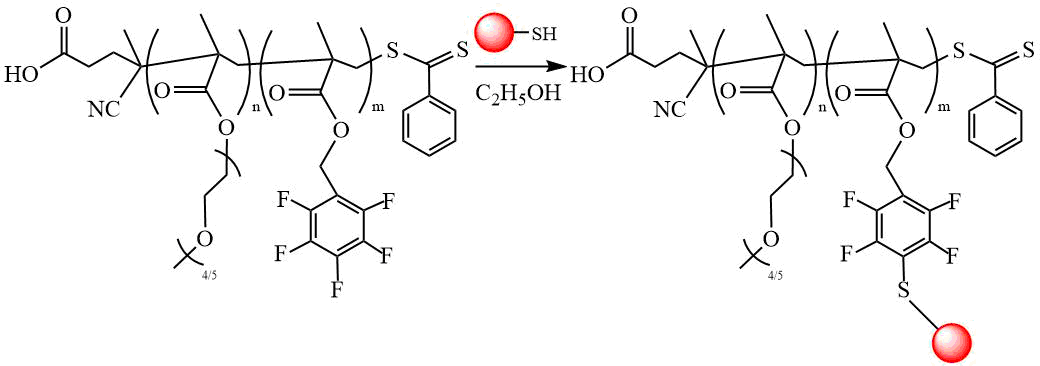
Scheme 17. Post-polymerization modification of DC PEGMA-PFBMA with various thiols [31].
Recently Grigoreva et al. published the results of a detailed study of RAFT polymerization of 2,2,3,3-tetrafluoropropyl methacrylate (TFPMA) in the presence of both low molecular CTAs (such as DDMAT, DBTC, 2-cyano-2-propyldodecyl trithiocarbonate (CPDT), S,S'-bis(methyl-2-isobutyrate)trithiocarbonate (MBTC), 1-cyano-1-methylethylphenyl(4-pyridinyl)dithiocarbamate (CMPC), 4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid (CDTPA), Dibenzyl carbonotrithioate (BTC)) and macroCTAs based on AA, methacrylic acid (MAA) and GMA [32]. For two CTAs (CPDT and CDTPA), the values of the chain transfer constants (Ctr) were determined; their values were 1.6 and 3.2 respectively. The obtained amphiphilic DCs based on macroCTAs had Mn = 8 ÷ 45 kDa and polydispersity Mw/Mn = 1.08 ÷ 1.31.
Not so long ago, we synthesized DC based on 2-hydroxyethyl methacrylate (HEMA) and PFHEMA in the presence of low molecular weight CPDB in DMF at 60ºС (Scheme 18) and studied the surface properties of DC coatings on cotton fabric depending on the DC composition [33]. It was found that the transformation of hydrophobic coatings into superhydrophobic ones is determined by the composition of the DC and occurs when the ratio of lengths of the PHEMA and PPFHEMA blocks PnPHEMA /Pn PPFHEMA = 15.12. The maximum improved surface properties are observed when applied to cotton fabric DC with composition of PHEMA:PPFHEMA = 6:94 mol.%; WCA and CA of diiodomethane θН2О = 152±2º and θCH2I2 = 120±3º, and these indicators practically do not change after repeated washing with washing powder at a temperature of 40ºС for 45 min.
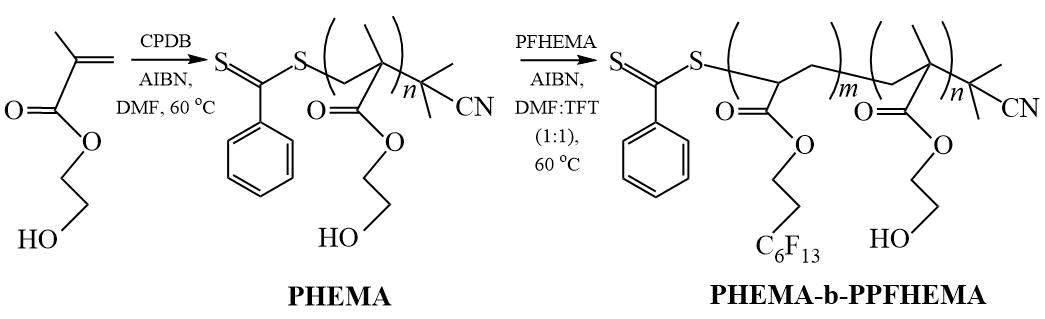
Scheme 18. Synthesis of DC PHEMA-b-PPFHEMA [33].
Conclusion
It can be seen from the presented review that over the past decade, thanks to the use of the RAFT polymerization method, significant progress has been made in the directed synthesis of new (co)polymers based on fluorinated (meth)acrylate to create materials with unique properties, in particular, superhydrophobic coatings of various surfaces.
Acknowledgments
This work was financially supported by the Russian Science Foundation (project no. 17-13-01359-P). Recording of the NMR spectra and elemental analysis were financial supported by the Ministry of Science and Higher Education of the Russian Federation using the equipment of Center for molecular composition studies of A.N. Nesmeyanov Institute of Organoelement Compounds, RAS.
References
- Park I. J., Lee S. B, Choi C. K., Surface properties for poly(perfluoroalkylethyl methacrylate) / poly(n‐alkyl methacrylate)s mixtures, J. Appl. Polym. Sci., 1994, 54(10), 1449-1454.
- Roche V., Vacandio F., Bertin D., Massiani Y., Corrosion performance of lamellae nanostructured fluorinated organic coating applied on steel, J. Electroceramics, 2006, 16(1), 41-47.
- Liu M. F., Chen Y. L., Zhang C., Bo Z. S., Stable superhydrophobic fluorine containing polyfluorenes, Chinese J. Polym. Sci., 2012, 30(2), 308-315.
- Souzy R., Ameduri B., Boutevin B., Synthesis and (co)polymerization of monofluoro, difluoro, trifluorostyrene and ((trifluorovinyl)oxy)benzene, Prog. Polym. Sci., 2004,. 29(3), 75-106.
- Natanya M.L. Hansen, Jankova K., Hvilsted S., Fluoropolymer materials and architectures prepared by controlled radical polymerizations, Eur. Polym. J., 2007, 43(2), 255-293.
- Bruno A., Controlled radical (Co)polymerization of fluoromonomers, Macromolecules 2010, V. 43. № 24. P. 10163-10184.
- Chiefari J., Chong Y. K., Ercole F., Krstina J., Jeffery J., Le T. P. T., Mayadunne R. T. A., Meijs G. F., Moad C. L., Moad G., Rizzardo E., Thang, S. H., Living free-radical polymerization by reversible addition−fragmentation chain transfer: The RAFT process, Macromolecules, 1998, 31(16), 5559-5562.
- Ma Z., Lacroix-Desmazes P., Synthesis of hydrophilic/CO2-philic poly(ethylene oxide)-b-poly(1,1,2,2-tetrahydroperfluorodecyl acrylate) block copolymers via controlled/living radical polymerizations and their properties in liquid and supercritical CO2, J. Polym. Sci. Part A Polym. Chem., 2004, 42(10), 2405-2415.
- Grignard B., Jérôme C., Calberg C., Detrembleur C., Jérôme R., Controlled synthesis of carboxylic acid end-capped poly(heptadecafluorodecyl acrylate) and copolymers with 2-hydroxyethyl acrylate, J. Polym. Sci. Part A Polym. Chem., 2007, 45(8), 1499-1506.
- Skrabania K., Laschewsky A., Berlepsch H., Böttcher C., Synthesis and micellar self-assembly of ternary hydrophilic- lipophilicfluorophilic block copolymers with a linear PEO chain, Langmuir, 2009, 25(13), 7594-7601.
- Yi F., Zheng S., Liu T., Nanostructures and surface hydrophobicity of self-assembled thermosets involving epoxy resin and poly(2,2,2-trifluoroethyl acrylate)-block- poly(ethylene oxide) amphiphilic diblock copolymer, J. Phys. Chem. B, 2009, 113(7), 1857-1868.
- Yi F., Yu R., Zheng S., Li X., Nanostructured thermosets from epoxy and poly(2,2,2-trifluoroethyl acrylate)-block-poly(glycidyl methacrylate) diblock copolymer: Demixing of reactive blocks and thermomechanical properties, Polymer, 2011, 52(24), 5669-5680.
- Li G., Zheng H., Wang Y., Wang H., Dong Q., Bai, R., A facile strategy for the fabrication of highly stable superhydrophobic cotton fabric using amphiphilic fluorinated triblock azide copolymers, Polymer, 2010, 51(9), 1940-1946.
- Koiry B. P., Klok H.-A., Singha N. K, Copolymerization of 2,2,3,3,4,4,4-heptafluorobutyl acrylate with butyl acrylate via RAFT polymerization, J. Fluor. Chem., 2014, 165, 109-115.
- Koiry B. P., Chakrabarty A., Singha N. K., Fluorinated amphiphilic block copolymers via RAFT polymerization and their application as surf-RAFT agent in miniemulsion polymerization, RSC Adv, 2015, 5(20), 15461-15468.
- Koiry B. P., Ponnupandian S., Choudhury S., Singha N. K., Syntheses and morphologies of fluorinated diblock copolymer prepared via RAFT polymerization, J. Fluor. Chem, 2016, 189, 51‑58.
- Azhar U., Huyan C., Wan X., Xu A., Li H., Geng B., Zhang S., A cationic fluorosurfactant for fabrication of high-performance fluoropolymer foams with controllable morphology, Mater. Des., 2017, 124, 194-202.
- Dai H., Yin G-Z., Zhao F-J., Bian Z-X., Zhang W-B., Miao X-R., Li H., Facile synthesis and hierarchical assembly of polystyrene-block- poly (perfluorooctylethyl acrylates, Polymer, 2017, 113, 46-52.
- Grigoreva A., Polozov E., Zaitsev S., Controlled synthesis and self-assembly of amphiphilic copolymers based on 2,2,3,3,4,4,5,5-octafluoropentyl acrylate and acrylic acid, Colloid Polym. Sci., 2019, 297(11–12), 1423-1435.
- Chiefari J., Mayadunne R.T.A., Moad C.L., Moad G., Rizzardo E., Postma A., Skidmore M.A., Thang S.H., Thiocarbonylthio compounds (S=C(Z)S-R) in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization). Effect of the activating Group Z, Macromolecules, 2003, 36(7), 2273-2283.
- Eberhardt M., Théato P., RAFT polymerization of pentafluorophenyl methacrylate: Preparation of reactive linear diblock copolymers, Macromol. Rapid Commun., 2005, 26(18), 1488-1493.
- Inoue Y., Watanabe J., Takai M., Yusa S. I., Ishihara K., Synthesis of sequence-controlled copolymers from extremely polar and apolar monomers by living radical polymerization and their phase-separated structures, J. Polym. Sci. Part A Polym. Chem., 2005, 43(23), 6073-6083.
- Guan C.-M., Luo Z.-H., Qiu J.-J., Tang P.-P., Novel fluorosilicone triblock copolymers prepared by two-step RAFT polymerization: Synthesis, characterization, and surface properties, Eur. Polym. J., 2010, 46(7), 1582-1593.
- Mya K. Y., Lin E. M. J., Gudipati C. S., Gose H. B. A. S., He C., Self-assembly of block copolymer micelles: Synthesis via reversible addition-fragmentation chain transfer polymerization and aqueous solution properties, J. Phys. Chem. B, 2010, 114(28), 9128-9134.
- Liu X., Chen J., Sun P., Liu Z.-W., Liu Z. T., Grafting modification of ramie fibers with poly(2,2,2-trifluoroethyl methacrylate) via reversible addition-fragmentation chain transfer (RAFT) polymerization in supercritical carbon dioxide, React. Funct. Polym., 2010, 70(12), 972-979.
- Li G., Xu A., Geng B., Yang S., Wu G., Zhang S., Synthesis and characterization of fluorinated diblock copolymer of 2,2,2-trifluoroethyl methacrylate and methyl methacrylate based on RAFT polymerization, J. Fluor. Chem., 2014, 165, 132-137.
- Huo M., Zeng M., Li D., Liu L., Wei Y., Yuan J., Tailoring the multicompartment nanostructures of fluoro-containing ABC triblock terpolymer assemblies via polymerization-induced self-assembly, Macromolecules, 2017, 50(20), 8212-8220.
- Huo M., Zhang Y., M. Zeng M., Liu L., Wei Y., Yuan J., Morphology evolution of polymeric assemblies regulated with fluoro-containing mesogen in polymerization-induced self-assembly, Macromolecules, 2017, 50(20), 8192-8201.
- Chakrabarty A., Ponnupandian S., Kang N. G., Mays J. W., Singha N. K., Designing superhydrophobic surface based on fluoropolymer–silica nanocomposite via RAFT-mediated polymerization-induced self-assembly, J. Polym. Sci. Part A Polym. Chem., 2018, 56(3), 266-275.
- Wang C., Li X., Deng H., Synthesis of a fluoromethacrylate hydroxystyrene block copolymer capable of rapidly forming sub-5 nm domains at low temperatures, ACS Macro Lett., 2019, 8(4), 368-373.
- Busatto N., Keddie J. L., Roth P. J., Sphere-to-worm morphological transitions and size changes through thiol-para-fluoro core modification of PISA-made nano-objects, Polym. Chem. 2020, 11(3), 704-711.
- Grigoreva A., Polozov E., Zaitsev S., Reversible addition-fragmentation chain transfer (RAFT) polymerization of 2,2,3,3-tetrafluoropropyl methacrylate: Kinetic and structural features, J. Fluor. Chem., 2019, 232, 109484.
- Chekurov K.E., Barabanova A. I., Blagodatskikh I. V., Kabaeva N. M., Barakovskaya I. G., Buyanovskaya A. G., Khokhlov A. R., Omniphobic coatings based on amphiphilic diblock copolymers of 2-(perfluorohexyl)ethyl methacrylate and 2-hydroxyethyl methacrylate, Dokl. Chem., 2021, 496(1), 18-23.
ARTICLE INFO
Received 24 June 2021
Accepted 29 June 2021
Available online August 2021
Recommended for publication by Prof. S. M. Igumnov
Fluorine Notes, 2021, 137, 1-2
