Received: September 2021
DOI 10.17677/fn20714807.2021.05.01
Fluorine Notes, 2021, 138, 1-2
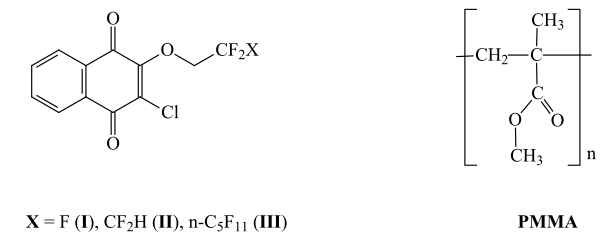
INFLUENCE OF 2-CHLORO-3-POLYFLUOROALKOXY-[1,4] -NAPHTHOQUINONES ON THERMAL OXIDATIVE STABILITY OF POLY(METHYL METHACRYLATE)
O. A. Melnik, M. I. Buzin, V. I. Dyachenko
A.N. Nesmeyanov Institute of Organoelement Compounds RAS, Russian Federation, 119991 Moscow, Vavilov st. 28.
e-mail: omel@ineos.ac.ru
Abstract: The polymers of methyl methacrylate with 2-chloro-3-polyfluoroalkoxy-[1,4]-naphthoquinones have been synthesized by free radical polymerization method. Their physicochemical properties and thermal characteristics have been studied. It has been shown that using of fluorine-containing [1,4]-naphthoquinones as aт antioxidant additives leads to a significant increase in thermal oxidative stability of poly(methyl methacrylate).
Key words: 2-chloro-3-polyfluoroalkoxy-[1,4]-naphthoquinones, poly(methyl methacrylate), free radical polymerization, heat resistance.
As a result of intensive development of new technologies, the requirements for polymer materials are rise sharply. The most common large-tonnage polymers include the polymers of esters of acrylic and methacrylic acids (for production of organic glasses, contact lenses, etc.) [1, 2]. Among them, a special place is occupied by poly(methyl methacrylate) (PMMA) - a transparent, impact-resistant material with good optical and dielectric properties, strong depletion effect and low water absorption [3, 4]. At the same time, PMMA, like other vinyl polymers, does not possess the required heat- and thermoresistance, which significantly narrows the scope of its practical application at elevated temperatures. To reduce the possibility of destructive processes or slow them down as much as possible, the stabilizers, various modifying additives and antioxidants are introduced into these polymers [3].
[1,4]-Naphthoquinones are one of the most important classes of organic compounds with antioxidant properties [5]. Among the derivatives of [1,4]-naphthoquinone, fluorine-containing [1,4]-naphthoquinones are of considerable interest; for this reason it can be used as precursors to obtain antioxidants [6, 7].
In this paper, the authors studied the stabilizing effect on thermal oxidative stability of PMMA 2-chloro-3-polyfluoroalkoxy-[1,4]-naphthoquinones IIII, which have different lengths of polyfluoroalkoxy radical (OCH2CF3, OCH2C2F4H, OCH2C6F13). Compounds I-III are low-melting, stable, well-soluble in organic solvents substances, that can be obtained by interaction of commercially available 2,3-dichloro-[1,4]-naphthoquine and fluorinated alcohols, manufactured commercially [8]. The presence of fluorine-containing substituents in their molecules has a positive effect on solubility in unsaturated monomers, such as alkyl(meth)acrylates, styrene, vinyl acetate, etc.
Earlier, it was shown by cyclic voltammetry method that 2-chloro-3-polyfluoroalkoxy-[1,4]-naphthoquinones, studied by authors in aprotic medium, easily undergo stepwise reversible electroreduction (the reduction potential Е01=-0,35 V) [9]. This indicates the possibility of using compounds I - III for slow down the thermal oxidative degradation of polymers by neutralizing the resulting radicals.
For experimental confirmation of this assumption, PMMA samples with additives of compounds I, II, III were synthesized and their thermal characteristics were studied. Free radical polymerization of methyl methacrylate in mass was carried out in evacuated, sealed glass ampoules in the presence of 0.5wt.% of initiating agent - azobisisobutyric acid dinitrile - at 60°C. The polymers are solid transparent glassy yellow samples; its structure was established by IR and mass spectroscopy. The IR spectrum of PMMA with I contains the absorption bands specific to units of both methyl methacrylate and compound I: 710, 717, 1663, 1676 сm-1 (fragments of [1,4]-naphthoquinone); 1142 and 1190 сm-1 (CF3); 1719 сm-1 (С=О methyl methacrylate). At the same time, there are no absorption bands of stretching vibrations of C=C bond at 1645 С=О, which were present in IR spectrum of starting methyl methacrylate. IR spectrum of PMMA with II also contains the absorption bands specific to [1,4]-naphthoquinone (709, 716, 1661, 1677 сm-1) and intense bands at 1130 and 1148 сm-1 corresponding to СF2 group. IR spectrum of PMMA with III contains intense bands at 1127 and 1145 cm-1 (СF2), and at 1186 and 1198 cm-1 (СF3).
Mass spectral study of methyl methacrylate polymer with 1 mol.% of I by direct injection method at ionization energy of 70 eV showed the presence of characteristic traces of ionic destruction of PMMA, m/z, (%): 100 [M]+ (54), 85 (8), 69 (92), 59 (16), 41 (100), 29 (12), 15 (18) (see Fig. 1). Mass spectrum also contains the molecular ion 290[M]+ 2-chloro-3-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone I and characteristic traces of its destruction as a result of electron impact: 270, 221 , 157, 151, 129, 123, 76, 50 and 18. Due to low concentration of compound I, their intensity does not exceed 6%.
It is known that one of the main characteristics of polymers that determine their scope of performance are the glass transition temperature Тg and also the temperature of destruction onset Тd [10, 11]. It should be noted that for comparison, we tested PMMA synthesized without additives and under similar conditions. Evaluation of heat resistance of obtained samples showed that the introduction of I - III leads to a decrease in Тg of PMMA, apparently due to polymer loosening by bulky groups. Thus, Тg of PMMA and PMMA with 1 mol.% of compound I are 105 and 102°C, respectively. With increase in the content of I to 3 mol.%, Тg of polymer is equal 90°C.
The thermoresistance of synthesized polymers was evaluated by temperature of decomposition onset, which was taken to be the temperature at which the weight loss of analyzed sample was 10% of initial one. It was determined by dynamic thermogravimetric analysis (TGA) at a heating rate of 10 °C/min in air. We have shown a significant improvement in thermoresistance of PMMA when compounds I – III are used as additives (see Figs. 1, 2). It was found that Td of polymers with I - III increases as increases in the content of fluorine-containing [1,4]‑naphthoquinones. For example, Td of PMMA is 265°C. As can be seen from Fig. 1, Td of PMMA containing 1 mol.% of compound I is equal 305°C, while for the sample with 3 mol.% I it is equal 320°C.
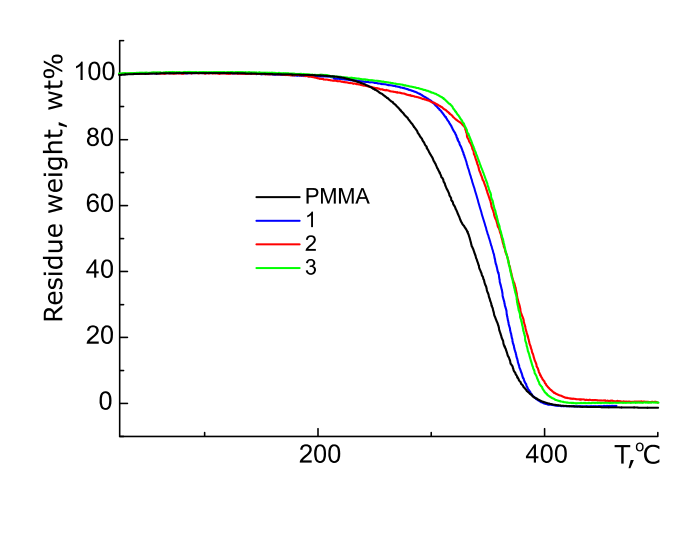
Figure 1. TGA curves of PMMA and PMMA with 1, 2, and 3 mol. % of compound I.
Td of PMMA samples with additions of 1 mol. % of compounds II and III is 306°C, i.e. its value in this case does not depend on the length of polyfluoroalkyl substituent. For PMMA samples containing 2 mol. % I–III, the thermoresistance is higher for polymer with II (see Fig. 2).
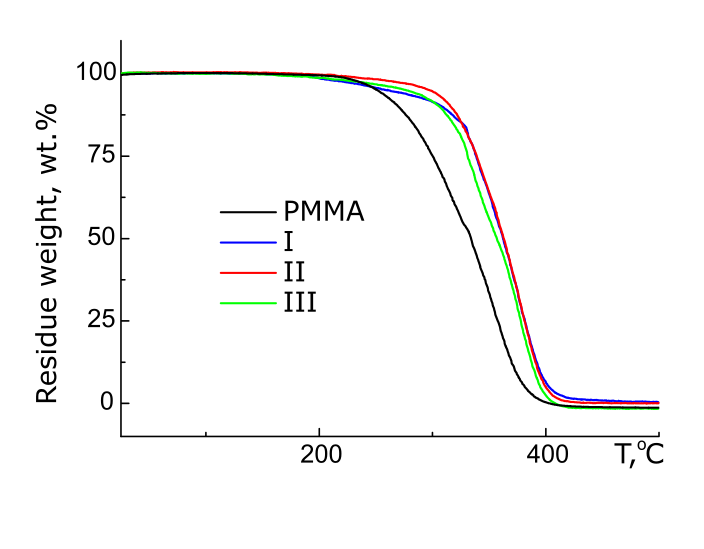
Figure 2. TGA curves of PMMA and PMMA with 2 mol. % of compounds I, II, III.
Thus, a comparison of obtained results with TGA PMMA data indicates a significant improvement in thermal oxidative stability of polymer when fluorine-containing [1,4]‑naphthoquinones I–III are used as additives. Thermoresistance of PMMA samples with small (1–3 mol. %) content of I-III exceeds the thermal resistance of PMMA by 40-55°C.
Experimental part
1Н and 19F NMR spectra were recorded in CDCl3 via Bruker Avance 400 spectrometer (at frequence 400 and 376 MHz, respectively). When recording 1H NMR spectra, Me4Si was used as an internal standard, 19F NMR spectra of compounds I, II СF3CO2H as an external standard, 19F NMR spectra of compound III СFCl3 (as an external standard). IR spectra were recorded via Bruker Vertex 70 v Fourier spectrometer with resolution of 4 cm-1 in frustrated total internal reflection mode using PIKE Glady ATR attachment with diamond working element. Mass spectra were recorded via Finnigan MAT INCOS 50 quadrupole mass spectrometer (with direct input and ionization energy 70 eV). The glass transition temperature of polymers was determined by thermomechanical analysis via TMA Q400 analyser manufactured by TA Instruments (with probe 2.54 mm in diameter and weighting 100 g) at a sample heating rate of 5°C/min in temperature range 20–250°C. Dynamic thermogravimetric analysis was carried out via Q-1500 derivatograph manufactured by MOM.
2-Chloro-3-polyfluoroalkoxy-[1,4]-naphthoquinones (I, II, III) were synthesized as described in [8].
2-Chloro-3-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (I)
M. p. 105-106 °C. Founded, %: C, 50.07; H, 1.98; F, 19.29. C12H6ClF3O3. Calculated, %: C, 49.59; H, 2.08; F, 19.61. 1H NMR (CDCl3, δ, ppm, J/Hz): 8.18 (m, 1H, Ar), 8.16 (m, 1H, Ar), 7.81 (m, 2H, Ar) - ABCD system; 4.93 (q, 2H, OCH2, 3JH-F=8). 19F NMR (CDCl3, δ, ppm, J/Hz): 2.77 (s, 3F, CF3).
2-Chloro-3-(2,2,3,3-tetrafluoropropoxy)-[1,4]-naphthoquinone (II)
M. p. 119-120 °C. Founded, %: C, 48.58; H, 2.08; F, 23.22. C13H7ClF4O3. Calculated, %: C, 48.40; H, 2.19; F, 23.55. 1H NMR (CDCl3, δ, ppm, J/Hz): 8.19 (m, 1H, Ar), 8.13 (s, 1H, Ar), 7.81 (m, 2H, Ar) - ABCD system; 6.21 (tt 1H, CF2H, 2JH-F = 52, 3JH-F = 4); 4.94 (t, 2H, OCH2, 3JH-F = 11). 19F NMR (CDCl3, δ, ppm, J/Hz): -48.21 (s, 2F, CF2); -61.66 (s, 2F, CF2).
2-Chloro-3-(2,2,3,3,4,4,5,5,6,6,7,7,7-tridecafluoroheptyloxy)-[1,4]-naphthoquinone (III)
M. p. 82-83 °C. Founded, %: C, 38.13; H, 1.28; F, 46.04. C17H6ClF13O3. Calculated, %: C, 37.77; H, 1.12; F, 45.68. 1H NMR (CDCl3, δ, ppm, J/Hz): 8.19 (m, 1H, Ar), 8.13 (m, 1H, Ar), 7.82 (m, 2H, Ar) - ABCD system); 5.10 (t, 2H, OCH2, 3JH-F=11). 19F NMR (CDCl3, δ, ppm, J/Hz): -80.71 (t, 3 F, CF3, 3JH-F=11); -120.56 (td, 2 F, CF2, 3JF-F=11, 4JF-F = 4); -122.08 (m, 2F, CF2); -122.74 (m, 2F, CF2); -122.99 (m, 2F, CF2); -126.09 (t, 2 F, CF2, 3JF-F=15).
Preparation of PMMA with 2-chloro-3-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (I) in molar ratio of 99: 1
To a solution of 2.50 g of freshly distilled methyl methacrylate (Aldrich, 99%) and 0.072 g of 2-chloro-3-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (I) 0.013 g (0.5 wt. %) of azobisisobutyric acid dinitrile was added as a polymerization initiator. Prepared reaction mixture was filtered into a glass ampoule, which was then degassed by freezing three times by immersion in liquid nitrogen followed by thawing in a vacuum, sealed and placed in a thermostat. Polymerization temperature was 60°C. After 4 hours, the ampoule was removed, cooled and opened. The clear yellow solid polymer was dried in a vacuum at 40°C for 24 hours to constant weight.
Preparation of PMMA with 2-chloro-3-(2,2,3,3-tetrafluoropropoxy)-[1,4]-naphthoquinone (II) in molar ratio of 99: 1
Similar to the above described method, from 2.50 g of freshly distilled methyl methacrylate and 0.081 g of 2-chloro-3-(2,2,3,3-tetrafluoropropoxy)-1,4] -naphthoquinone (II) are prepared.
Preparation of PMMA with 2-chloro-3-(2,2,3,3,4,4,5,5,6,6,7,7,7-tridecafluoroheptyloxy)-[1,4]-naphthoquinone (III) in molar ratio 99: 1
Similar to the above described method, from 3.30 g of freshly distilled methyl methacrylate and 0.180 g of 2-chloro-3-(2,2,3,3,4,4,5,5,6,6,7,7,7-tridecafluoroheptyloxy)-[1,4]-naphthoquinone (III) are prepared.
We also synthesized samples of PMMA with compounds I - III at a molar ratio of 98: 2 and 97: 3.
Acknowledgments
This work was supported by Ministry of Science and Higher Education of Russian Federation using the scientific equipment of the Center for Study of Molecular Structures of INEOS RAS.
The authors are grateful to M.G. Ezernitskaya for registration IR spectra, and E.S. Afanasyev - for performing the thermomechanical analysis of polymer samples.
References
- Technology of plastics, Korshak V.V. ed., 3rd ed., Moscow, Khimiya, 1985, 560 pp. (in Russian)
- Kireev V.V., High molecular weight compounds, Moscow, Vysshaya shkola, 1992, 512 pp. (in Russian)
- Technical properties of polymeric materials, Kryzhanovsky V.C. ed., 2nd ed. S-Pb., Professiya, 2005, 235 pp. (in Russian)
- Debsky V., Poly(methyl methacrylate), Moscow, Khimiya, 1972, 152 pp. (in Russian)
- Thomson R.H., Naturally occurring quinones IV: Recent advances, London: Springer, 1997, 746 pp.
- Zhang Y. et al., Org. Lett., 2017, 19 (6), p. 1302-1305.
- Paul K. et al., Org. Lett., 2009, 11 (20), 4728-4731.
- Dyachenko V.I., Fluorine notes, 2021, 134 (1).
- Peregudova S.M., Dyachenko V.I., Fluorine notes, 2021, 135 (2).
- Askadsky A.A., Khokhlov A.R., Introduction to physicochemistry of polymers. Moscow, Nauchnyj Mir, 2009, 380 pp. (in Russian)
- Askadsky A.A., Popova M.N., Kondrashchenko V.I., Physicochemistry of polymeric materials and methods of their research, Moscow, ASV, 2015, 408 pp. (in Russian)
ARTICLE INFO
Received 17 September 2021
Accepted 22 September 2021
Available online October 2021
Recommended for publication by PhD M. A. Manaenkova
Fluorine Notes, 2021, 138, 1-2
