Received: September 2021
DOI 10.17677/fn20714807.2021.05.02
Fluorine Notes, 2021, 138, 3-4
MECHANISM FOR INITIATION CATION POLYMERIZATION OF p-METYLSTYRENE IN THE PRESENCE OF BF3∙HF CATALYST IN TOLUENE AT 1: 1: 3 RATIO
1V.A. Babkin, 1D.S. Andreev, A.V. Ignatov1, S.E. Karpushova1, E.S. Titova2, 3, A.I. Rakhimov2, V.T. Fomichev2
1Volgograd State Technical University (Sebryakovsky br.), 403343 Volgograd Region, Mikhailovka,
Michurina st., 21.
e-mail: babkin_v.a@mail.ru
2Volgograd State Technical University, 400005 Volgograd, Lenin av., 28.
e-mail: titova051@rambler.ru
3Volgograd State Medical University, 400131 Pavshikh Bortsov sq., 1.
e-mail: titova051@rambler.ru
Abstract: In this paper, initiation mechanism of cationic polymerization of p-methylstyrene in the presence of a complex catalyst BF3 ∙ HF in toluene at the ratio of 1: 1: 3 has been studied by ab initio method. The values of activation energies and reaction enthalpy are estimated.
Keywords: initiation mechanism, p-methylstyrene, boron fluoride catalyst - hydrogen fluoride, toluene, activation energy, enthalpy, ab initio method.
Introduction
Boron fluoride - hydrogen fluoride (BF3∙HF) is a typical catalyst for cationic polymerization [1], the classical stages of which are initiation, growth and termination of material chain [2]. It is obvious that varying the character of Lewis acid (for example, BF3, BF2CH3, BF(CH3)2, B(CH3)3, BF2CH5, etc.) and Brønsted acid (HF, HCl, HBr, etc.) in catalyst composition, as well as the stoichiometric composition “catalyst : solvent” (1: 1 (in this case - toluene), 1: 2, 1: 3, 1: 4, etc.) opens up in practice the possibility of controlling the polymerization process at initiation stage, up to obtaining a polymer (oligomer, telomer, and, in particular - poly-p-methylstyrene) with specified physicochemical properties. A number of important fundamental issues concerning the mechanisms of elementary acts of cationic polymerization of p-methylstyrene: initiation, growth and termination of a chain in the presence of BF3 ∙ HF catalyst in toluene, remain unclear until now. And, in particular, the elucidation of energy dependence of initiation reaction for cationic polymerization of p-methylstyrene (EA is activation energy, ET is heat effect of reaction) on stoichiometric composition of molecular system “catalyst – solvent” (BF3 ∙ HF - toluene 1: 1, 1: 2, 1 : 3, 1: 4, etc.). The calculation of initiation mechanism of of stoichiometric compositions 1: 1 and 1: 2 (catalyst: solvent) was carried out in [3, 4]; therefore, the purpose of this paper is to study of initiation mechanism in the presence of this catalyst by calculating the reaction of interaction of monomer with initiator along RC1-H20 coordinate in toluene with stoichiometric composition 1: 3 (within the framework of molecular model).
Methodical part
A quantum chemical study of initiation mechanism of p-methylstyrene was carried out by ab initio RHF/6-311G** method [5] in accordance with procedure, for example, described in [6 - 9], using software [10 - 12]. The reaction coordinate is RC(1)H(20).
Calculation results
The results of quantum chemical calculations (the initial model, the formed active center (AC), the energy profile of reaction, and the change in charges on the atoms directly involved in this reaction) are shown in Fig. 1-4 and in Table 1.
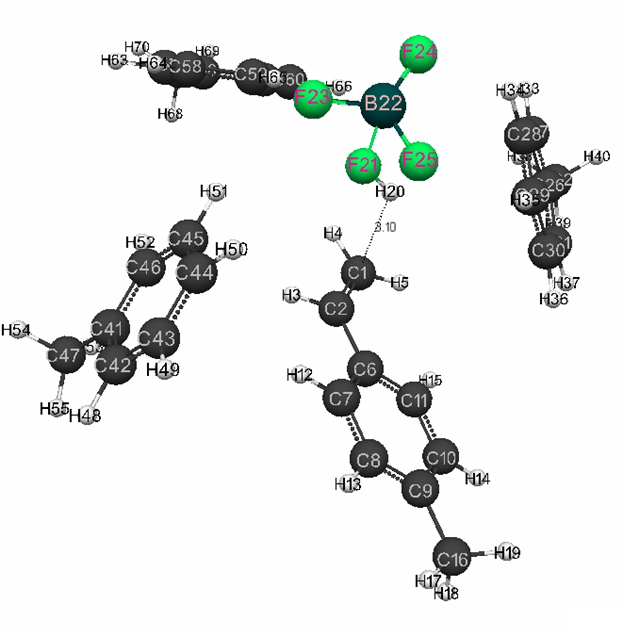
Figure 1. Model before interaction of complex catalyst HF · BF3 with p-methylstyrene in toluene with stoichiometric composition 1: 1: 3.
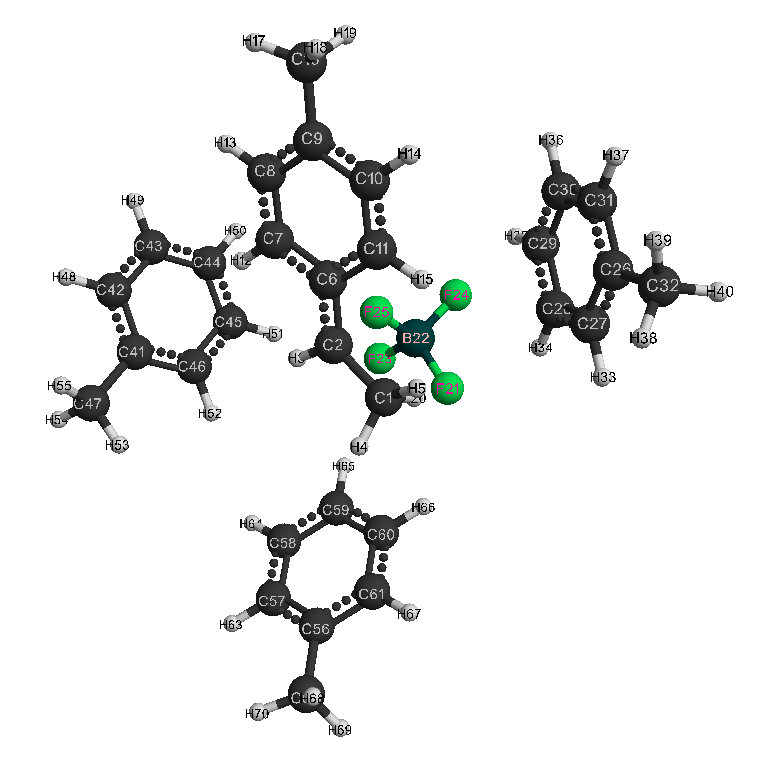
Figure 2. Model after reaction of HF · BF3 complex catalyst with p-methylstyrene in toluene with stoichiometric composition 1: 1: 3.
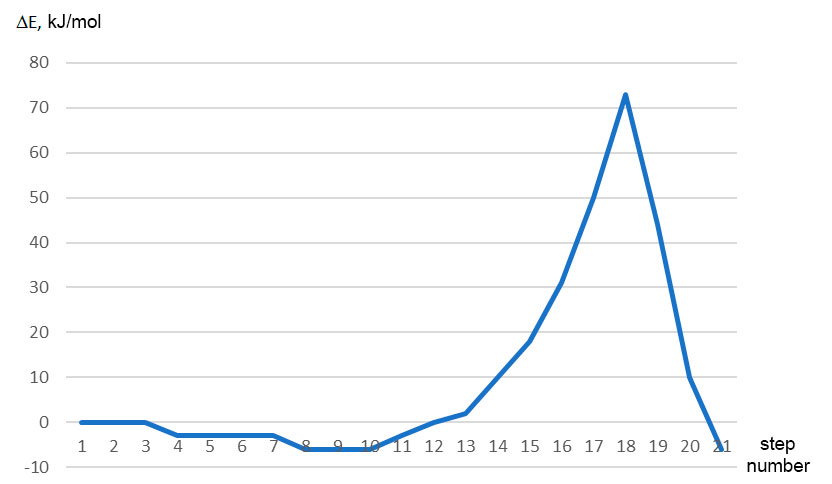
Figure 3. Change in total energy (E) along the coordinate of reaction studied (No. 1-21 is interaction steps).
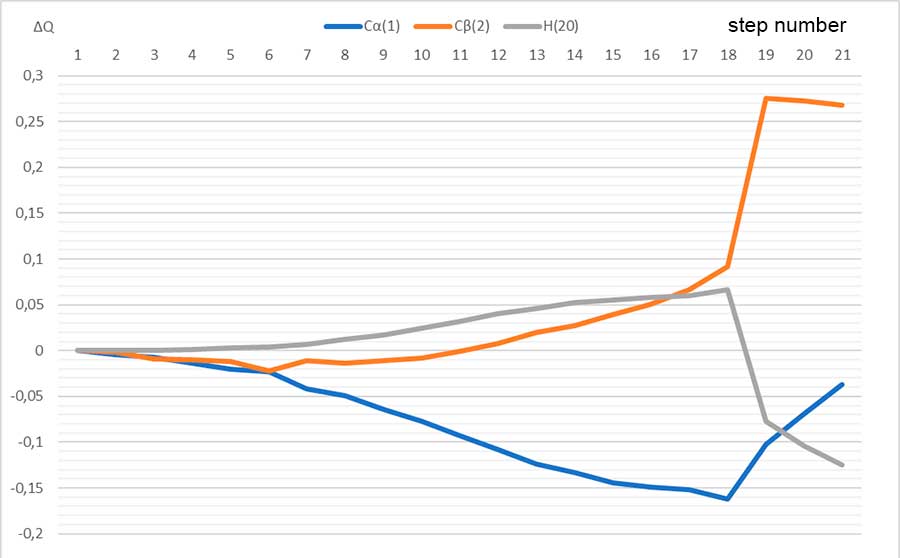
Figure 4. Changes in charges on atoms directly involved in reaction: C (1), C (2), H (20)
Table 1 shows the values of all charges on the atoms of molecular system at extremum points along the reaction coordinate RH(20)-C(1). From Table 1 it can be seen that the law of charge conservation clearly runs at each stage of components interaction.
Table 1. Atoms charges at extremum points (steps 1, 9, 18, 21).
|
Atom |
No. of step |
|||
|
1 |
9 |
18 |
21 |
|
|
C(1) |
-0,217 |
-0,281 |
-0,379 |
-0,254 |
|
C(2) |
-0,148 |
-0,159 |
-0,056 |
0,120 |
|
H(3) |
0,125 |
0,130 |
0,142 |
0,201 |
|
H(4) |
0,128 |
0,143 |
0,157 |
0,135 |
|
H(5) |
0,105 |
0,131 |
0,157 |
0,113 |
|
C(6) |
-0,080 |
-0,089 |
-0,127 |
-0,204 |
|
C(7) |
-0,066 |
-0,051 |
-0,025 |
0,063 |
|
C(8) |
-0,096 |
-0,098 |
-0,102 |
-0,139 |
|
C(9) |
-0,120 |
-0,115 |
-0,103 |
-0,046 |
|
C(10) |
-0,095 |
-0,096 |
-0,100 |
-0,120 |
|
C(11) |
-0,057 |
-0,044 |
-0,027 |
0,049 |
|
H(12) |
0,133 |
0,138 |
0,139 |
0,191 |
|
H(13) |
0,084 |
0,085 |
0,090 |
0,110 |
|
H(14) |
0,083 |
0,085 |
0,092 |
0,111 |
|
H(15) |
0,088 |
0,099 |
0,112 |
0,153 |
|
C(16) |
-0,176 |
-0,177 |
-0,179 |
-0,187 |
|
H(17) |
0,094 |
0,095 |
0,098 |
0,114 |
|
H(18) |
0,106 |
0,108 |
0,111 |
0,122 |
|
H(19) |
0,106 |
0,107 |
0,115 |
0,140 |
|
H(20) |
0,358 |
0,375 |
0,424 |
0,233 |
|
F(21) |
-0,339 |
-0,362 |
-0,479 |
-0,434 |
|
B(22) |
0,827 |
0,826 |
0,873 |
0,847 |
|
F(23) |
-0,272 |
-0,278 |
-0,305 |
-0,402 |
|
F(24) |
-0,276 |
-0,273 |
-0,298 |
-0,391 |
|
F(25) |
-0,276 |
-0,277 |
-0,307 |
-0,472 |
|
C(26) |
-0,120 |
-0,120 |
-0,121 |
-0,124 |
|
C(27) |
-0,094 |
-0,100 |
-0,099 |
-0,124 |
|
C(28) |
-0,104 |
-0,092 |
-0,098 |
-0,114 |
|
C(29) |
-0,180 |
-0,130 |
-0,136 |
-0,126 |
|
C(30) |
-0,060 |
-0,088 |
-0,091 |
-0,086 |
|
C(31) |
-0,100 |
-0,100 |
-0,095 |
-0,114 |
|
C(32) |
-0,177 |
-0,177 |
-0,177 |
-0,172 |
|
H(33) |
0,090 |
0,086 |
0,086 |
0,085 |
|
H(34) |
0,117 |
0,113 |
0,123 |
0,144 |
|
H(35) |
0,118 |
0,110 |
0,116 |
0,144 |
|
H(36) |
0,106 |
0,097 |
0,093 |
0,091 |
|
H(37) |
0,087 |
0,085 |
0,084 |
0,080 |
|
H(38) |
0,103 |
0,098 |
0,098 |
0,093 |
|
H(39) |
0,094 |
0,096 |
0,094 |
0,090 |
|
H(40) |
0,113 |
0,112 |
0,112 |
0,115 |
|
C(41) |
-0,114 |
-0,111 |
-0,112 |
-0,116 |
|
C(42) |
-0,099 |
-0,108 |
-0,106 |
-0,118 |
|
C(43) |
-0,083 |
-0,083 |
-0,086 |
-0,091 |
|
C(44) |
-0,127 |
-0,120 |
-0,122 |
-0,137 |
|
C(45) |
-0,093 |
-0,097 |
-0,106 |
-0,084 |
|
C(46) |
-0,119 |
-0,123 |
-0,126 |
-0,170 |
|
C(47) |
-0,176 |
-0,175 |
-0,175 |
-0,173 |
|
H(48) |
0,084 |
0,086 |
0,086 |
0,084 |
|
H(49) |
0,093 |
0,095 |
0,096 |
0,094 |
|
H(50) |
0,100 |
0,103 |
0,110 |
0,106 |
|
H(51) |
0,122 |
0,120 |
0,132 |
0,166 |
|
H(52) |
0,094 |
0,095 |
0,094 |
0,116 |
|
H(53) |
0,102 |
0,098 |
0,097 |
0,097 |
|
H(54) |
0,110 |
0,112 |
0,113 |
0,116 |
|
H(55) |
0,095 |
0,098 |
0,097 |
0,094 |
|
C(56) |
-0,123 |
-0,122 |
-0,123 |
-0,126 |
|
C(57) |
-0,091 |
-0,092 |
-0,092 |
-0,093 |
|
C(58) |
-0,094 |
-0,091 |
-0,091 |
-0,101 |
|
C(59) |
-0,108 |
-0,115 |
-0,119 |
-0,136 |
|
C(60) |
-0,100 |
-0,100 |
-0,105 |
-0,112 |
|
C(61) |
-0,095 |
-0,099 |
-0,099 |
-0,106 |
|
C(62) |
-0,178 |
-0,178 |
-0,177 |
-0,176 |
|
H(63) |
0,086 |
0,086 |
0,085 |
0,081 |
|
H(64) |
0,098 |
0,098 |
0,098 |
0,095 |
|
H(65) |
0,118 |
0,114 |
0,122 |
0,152 |
|
H(66) |
0,096 |
0,105 |
0,110 |
0,131 |
|
H(67) |
0,084 |
0,086 |
0,085 |
0,080 |
|
H(68) |
0,110 |
0,109 |
0,107 |
0,096 |
|
H(69) |
0,098 |
0,102 |
0,103 |
0,109 |
|
H(70) |
0,097 |
0,096 |
0,094 |
0,090 |
Thus, in this paper we performed the quantum chemical study of initiation mechanism of cationic polymerization of p-methylstyrene under the action of complex catalyst BF3 ∙ HF with toluene in the 1: 1: 3 ratio by ab initio method. Analysis of change in charges on atoms directly involved in this reaction (see Fig. 4), behavior of reaction fragments, breaking and formation of new bonds indicate that mechanism under study is usual acceptance of H (1)+ proton from BF3 ∙ HF catalyst and its addition to α-carbon monomer atom. The calculated values EA = 73 kJ/mol, ET = -6 kJ/mol.
References
- Kennedy, J., Cationic polymerization of olefins. Mir Publishing House, Moscow, 1978, 431 p. (in Russian)
- Odian, G., Principles of polymerization, Mir Publishing House, Moscow, 1974, 614 р. (in Russian)
- Babkin, V.А., Andreev D.S., Ignatov А.V. and others, Calculation of interaction mechanism for complex catalyst HF BF3 with p-methylstyrene in toluene with stoichiometric composition 1:1:1 by ab initio method, Fluorine Notes, 2021, 2(135), 3-4.
- Babkin, V.А., Andreev D.S., Ignatov А.V. and others, Mechanism for initiation cation polymerization of p-metylstyrene in the presence of BF3 ∙ HF catalyst in toluene at 1: 1: 2 ratio, Fluorine Notes. 2021, 3(136), 5-6.
- Cirelson V.G., Quantum Chemistry. Molecules, molecular systems and solids, Moscow, Publishing House «Binom», 2010, 496 p. (in Russian)
- V. A. Babkin and others, Quantum-chemical research of the interaction mechanism of the complex catalyst chloride aluminium-hydrochloric acid and p-methylstyrene in toluene by the ab initio method, Oxidation Communications, 2020, 43(2), 171-176.
- V. A. Babkin and others, Quantum chemical calculation of initiation mechanism of cationic polymerisation of propylene with chloride-aluminium aquacomplex, Oxidation Communications, 2020, 43(1), 24-29.
- V. A. Babkin and others, Quantum chemical investigation of the initiation mechanism of the cationic polymerisation of 4-methylpentene-1 with chloride-aluminum aquacomplex, Oxidation Communications, 2019, 42(3),275-281.
- V. A. Babkin and others, On the mechanism of cationic polymerisation of p-isopropylstyrene in the presence of a complex catalyst boron fluoride-water, Oxidation Communications, 2019, 42(1), 56-62.
- Granovsky, A. A., Firefly version 8, 2013. http://classic.chem.msu.su/gran/firefly/index.html
- M.W. Schmidt and others, General Atomic and Molecular Electronic Structure System, J. Comput. Chem., 1993, 14, 1347-1363.
- B.M. Bode, M.S. Gordon, MacMolPlt: A Graphical User Interface for GAMESS, Journal of Molecular Graphics, 1998, 16, 133-138.
ARTICLE INFO
Received 10 September 2021
Accepted 15 September 2021
Available online October 2021
Recommended for publication by PhD V. Don
Fluorine Notes, 2021, 138, 3-4
