Received: September 2021
DOI 10.17677/fn20714807.2021.05.03
Fluorine Notes, 2021, 138, 5-6
IONIC SERIES IN MASS SPECTRA OF TRIFLUOROMETHYL-SUBSTITUTED HETEROCYCLES AND CYCLOPROPANES NOT CONTAINING REGULAR FRAGMENT GROUPS
N. D. Kagramanov *, A.L. Sigan, A.S. Golubev
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: In the mass spectra of n-alkanes, n-perfluoroalkanes, n-carboxylic
acids, their methyl esters, cycloalkanes and perfluoropolycycloalkanes containing regular fragment
groups (C2H4)n or (CF2)n, all parallel series
of ions formed in parallel are quite easily determined by reference separations .
This research
presents a series of ions of mass spectra of trifluoromethyl-substituted compounds, that do not contain
regular fragment groups. The absence of regular fragment groups makes it difficult to establish chains
of ionic series, since characteristic detachments (-C2H4 and –CF2)
do not occur. As examples, the ionic series of mass spectra of three ethyl-5-aryl-5-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylates,
two 3-(trifluoromethyl)-2,1-benzisoxazoles, and eleven trans-2 -aryl-2-trifluoromethyl-1-nitrocyclopropanes.
During the fragmentation of ethyl 5-aryl-5-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylate derivatives,
two series are formed, leading to two stable fragment ions PhC+=O and PhC+=CH2.
Depending on the position of the substituent in relation to the CF3 group, either the
release of .CF3, or the rearrangement of CF2 is
recorded in the mass spectra of 3- (trifluoromethyl) -2,1-benzisoxazole derivatives. In the spectra
of trans-2-aryl-2-trifluoromethyl-1-nitrocyclopropanes, with the synchronous abstraction of HNO,
.CF3, and C2H2, appears an intense peak
of the rearrangement ion Ar-+C=O. Another way of fragmentation of 2-trifluoromethyl-1-nitrocyclopropane
derivatives is the formation of the trifluoronitromethane cation-radical [CF3NO2]+. with m/z 115, due to the release of neutral Aryl-C3H3 molecules, in which cyclopropene
is retained, but only without electron-withdrawing substituents. In the mass spectra of trans-2-aryl-2-trifluoromethyl-1-nitrocyclopropanes
containing substituents in the para- or meta- position of the phenyl group, the peaks intensities
of competing ionic series vary depending on the para- or meta- position of the substituent.
Key words: ion series in mass spectra, series of ions without regular fragment groups, ethyl 5-aryl-5-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylates, 3- (trifluoromethyl) -2,1-benzisoxazoles, trans-2- aryl-2-trifluoromethyl-1-nitrocyclopropanes.
Introduction
In mass spectra of compounds that do not contain regular fragment groups (C2H4)n or (CF2)n, the establishment of fragmentation chains of ionic series is complicated by the absence of characteristic reference detachments [1]. With the multivariance of the composition of the detached radical, it can be reliably identified when recording a high-resolution spectrum. Nevertheless, the presence of an array of data on low-resolution spectra for derivatives with various isotopic substituents usually allows a well-founded conclusion about the composition of the removed radicals. Consideration of all options for the detachment of primary radicals, the establishment of the ionic series of the spectrum and their matching with each other is more productive than the description of the interpreted spectrum as a whole. When asymmetric molecules with substituents are ionized by electrons, the fragmentation process, usually, begins with the peripheral functional groups of the substituents, which break the symmetry of the more stable central fragment.
Experimental part
Mass spectra of derivatives of ethyl 5-aryl-5-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylates, 3- (trifluoromethyl) -2,1-benzisoxazoles and trans-2-aryl-2-trifluoromethyl-1-nitrocyclopropanes were obtained on a “Finnigan Polaris Q” spectrometer (ion trap, range 30‑700 Da, energy 70 eV, DIP direct injection method, ion source temperature 250C). In order to assess the possibility of thermal decomposition of derivatives of trans-2-aryl-2-trifluoromethyl-1-nitrocyclopropanes, the temperature of the ion source was decreased from 250 to 50C.
Ionic series of ethyl 5-aryl-5-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylates
Ethyl 5-aryl-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylates have the properties of plant growth regulators. Comparison of their activity with existing herbicides confirms the promise of their use in agrochemistry. A new, convenient method for their synthesis and separation, elemental analysis, as well as NMR spectra and mass spectra are presented in [2]. Figure 1-3 shows two series of ions present in the mass spectra of three ethyl 5-aryl-5-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylates.
One of the series, starting with the detachment of the .CF3 radical, leads to the base peaks R-C6H4+C=O. Another series, starting with the detachment of the ethoxy radical of the terminal ethoxycarbonyl group and ending with the detachment of the .CF3 radical, leads to peaks of medium intensity R-C6H4+C=CH2.
Thus, in the spectrum of ethyl 5-phenyl-5- (trifluoromethyl) -4,5-dihydroisooxazole-3-carboxylate after the release of .CF3, there is a sequential detachment of the ethylene molecule, CO2 and acetonitrile CH3CN m/z 41 with the formation of the basic ion of oxyphenylmethylium Ph-+C=O. In the case of detachment of the terminal ethoxycarbonyl group, the .CNO radical with m/z 42 is released and .CF3 is detached with the formation of the phenylethenyl ion Ph-+C=CH2 m/z 103 (50%).
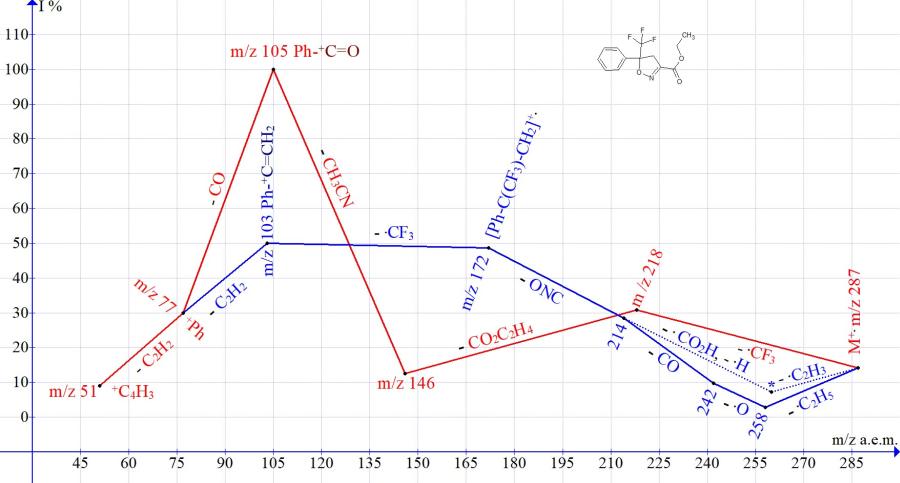
Figure 1. Mass spectrum ion series of ethyl 5-trifluoromethyl-5-phenyl-4,5-dihydroisoxazole-3-carboxylate C13H12F3NO3 MW: 287.
Similar ionic series are present in the mass spectra of ethyl 5-trifluoromethyl-5-para-tolyl-4,5-dihydroisoxazole-3-carboxylate (Fig. 2) and ethyl-5- (4-bromophenyl)-5-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylate (Fig. 3).
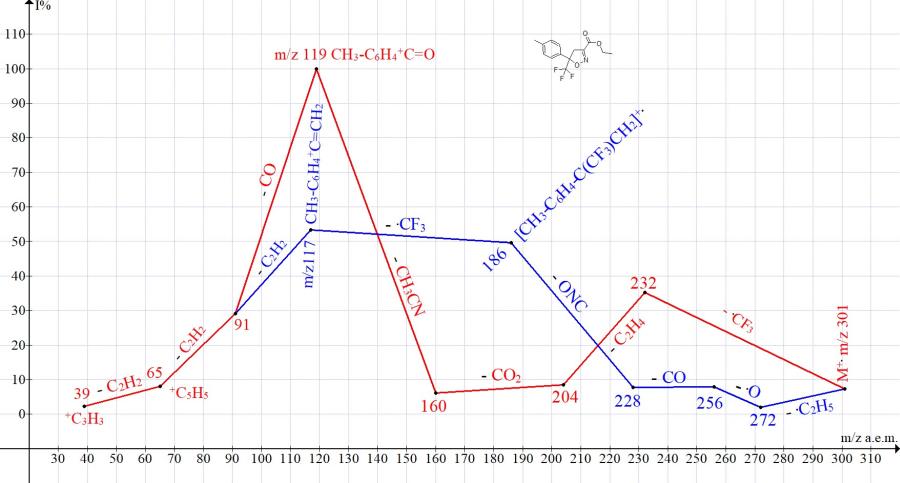
Figure 2. Mass spectrum ion series of ethyl 5-trifluoromethyl-5-p-tolyl-4,5-dihydroisoxazole-3-carboxylate C14H14F3NO3 MW: 301.
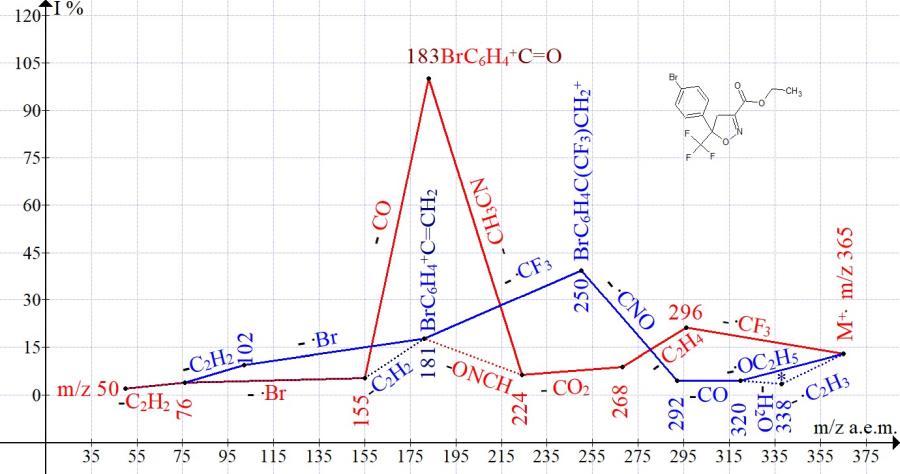
Figure 3. Mass spectrum ion series of ethyl 5- (4-bromophenyl) -5-trifluoromethyl-4,5- dihydroisoxazole-3-carboxylate C13H11BrF3NO3 MW: 365.
One of the series (Figs. 1-3), starting with the detachment of the .CF3 radical, leads to the basic peaks R-C6H4+C=O. Another series, starting with the detachment of the ethoxyl radical and ending with the detachmnet of the .CF3 radical, leads to peaks R-C6H4+C=CH2 of medium (Fig.1-2) and low intensity (Fig. 3).
Ionic series of 3- (trifluoromethyl) -2,1-benzisoxazoles
2,1-Benzisoxazoles (anthranils) are an important class of heterocyclic compounds used as synthons in organic synthesis. They are also of interest for biochemical research and pharmacology. In [3], a new synthesis method was proposed, and for the first time previously unknown 3- (trifluoromethyl) - and 3- (difluoromethyl) -2,1-benzisoxazoles were prepared.
In contrast to the spectra of ethyl 5-aryl-5-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylates [2], the spectra of less branched and more unsaturated structures of 3- (trifluoromethyl) -2,1-benzisoxazoles, depending on the mutual the positions of the substituents and the CF3 group, either the detachment of the trifluoromethyl group or rearrangement with the migration of the fluorine atom and the emission of CF2 is observed. In the mass spectrum of 3- (trifluoromethyl) -2,1-benzisoxazole, as well as in the spectra of its derivatives: 4-fluoro, 5-chloro, 5-bromo, and 7-nitro, the high intensity of the peaks (+.M-CF2) from 66% to 100%, corresponding to the detachment of difluorocarbene from trifluoromethyl groups [3]. Detachment of .F or HF occurs only as secondary processes, after the release of CF2.
The electron-withdrawing CF3 group weakens it with a double bond. When the molecule is ionized, one of the electrons of the weakened double bond 3a-3 is removed. Coordination of one of the fluorine atoms of the trifluoromethyl group with a new radical center-3а leads to rearrangement with the formation of a new C-F bond and the detachment of CF2.
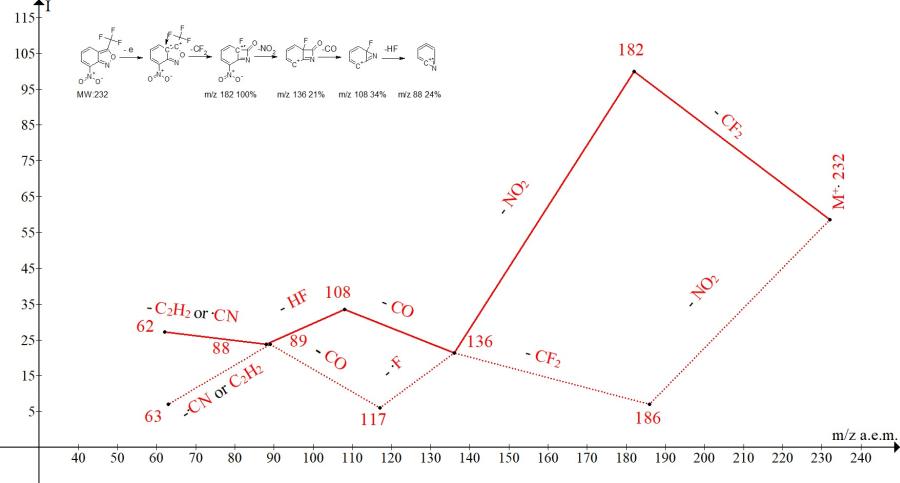
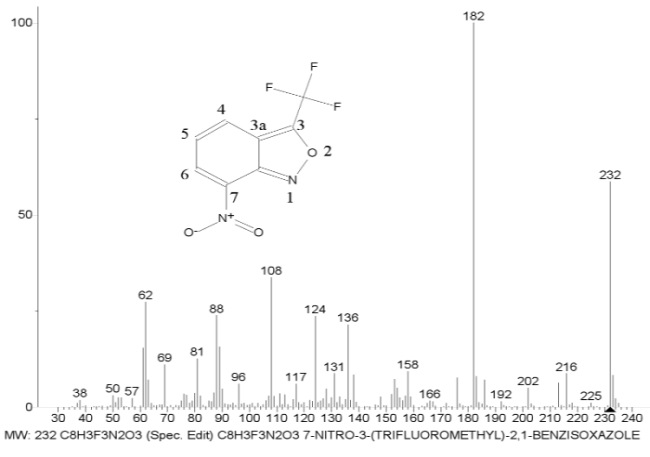
Figure 4. Mass spectrum of 7-nitro-3-(trifluoromethyl)-2,1-benzisoxazole C8H3F3N2O3 MW: 232 and two series of its ions.
Presented in Fig. 4, the two ion series of the mass spectrum differ only in the order of radical detachment and peak intensities. In essence, the less intense series of ions noted in Fig. 4. with the dotted line, is a mirror image of the more intense main series.
On the contrary, fragmentation of 4-substituted derivatives: 4-azido, 4-nitro, and, to a lesser extent, 4-fluoro-3- (trifluoromethyl) -2,1-benzisoxazole begins with the detachment of trifluoromethyl radicals. The ratio of the peaks intensities, corresponding to the detachment of .CF3 / CF2 in the spectra of benzisoxazoles with substituents 4-azido 100/0, 4-nitro 100/56, and 4-fluorine 40/66%, respectively.
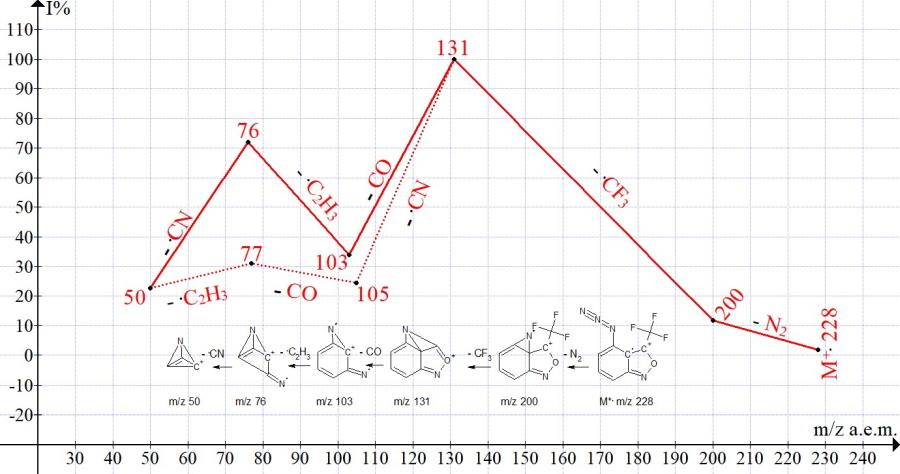
Figure 5. Mass spectrum ion series of 4-azido-3-(trifluoromethyl)-2,1-benzisoxazole
C8H3F3N4O MW: 228.
The azido group in the 4-th position, the closest to the double bond 3-3а, completely blocks the rearrangement detachment of the fluorine atom from the trifluoromethyl group. The substituents 4-nitro, and 4-fluorine, partially block the electron-withdrawing properties of the trifluoromethyl group, making it difficult to detach the fluorine atom.
In contrast to 4-azido-3-(trifluoromethyl) benzisoxazole, whose ionic series with 100% .CF3 detachment is shown in Fig. 5, competing fragmentation detachments occur in the spectra of 4-nitro- and 4-fluoro derivatives of 3- (trifluoromethyl) benzisoxazole .CF3 and CF2. It should be noted that there is no detachment of .CF3 in the spectrum of trifluoromethylbenzene (NIST #: 118781 ID #: 134414 DB: mainlib), but only successive emissions of CF2 and .F or .F and CF2 50/50% occur.
Ionic series of trans-2-aryl-2-trifluoromethyl-1-nitrocyclopropanes
Trans-2-aryl-2-trifluoromethyl-1-nitrocyclopropanes were first obtained and described in [4]. Their structure was confirmed by NMR, IR spectroscopy, mass spectrometry, X-ray structural and elemental analysis [4].
Since the cyclopropane ring has a high tension and low energy of C – C bond cleavage (54.4 kcal/mol) [5], the spectra of trans-2-aryl-2-trifluoromethyl-1-nitrocyclopropanes are examples of multivariance of decomposition. The features of the dissociative ionization of arylcyclopropanes are determined by the conjugation of the three-membered ring with the aromatic system [6,7], as well as by the substituents of the cyclopropane and the aryl group. In the case of cyclopropanes with electron-withdrawing substituents NO2 [8] and CF3, the tension of the cycle increases. By recording the spectra of 2-aryl-2-trifluoromethyl-1-nitrocyclopropane derivatives, carried out at ion source temperatures of 250 oC and 50 oC, it was confirmed that the fragmentation processes under consideration, are not associated with the thermal decomposition of the analytes in the ion source.
Fragmentation of 2-aryl-2-trifluoromethyl-1-nitrocyclopropanes, usually, begins with the detachment of HNO2 and CF3 with the formation of the cyclopropene ion Ar-+C3H2. Another decomposition path includes the synchronous detachment of HNO, .CF3, and C2H2 with the formation of a rearrangement ion Ar-+C=O, which is formed due to the migration of the oxygen atom NO2 during the detachment of HNO. Another common process specific for the fragmentation of 2-aryl-2-trifluoromethyl-1-nitrocyclopropanes is the formation of the ion peak with m/z 115 - the radical cation of trifluoronitromethane [CF3N2]+. . The main pathways for the decomposition of 2-aryl-2-trifluoromethyl-1-nitrocyclopropanes are shown in Scheme 1.
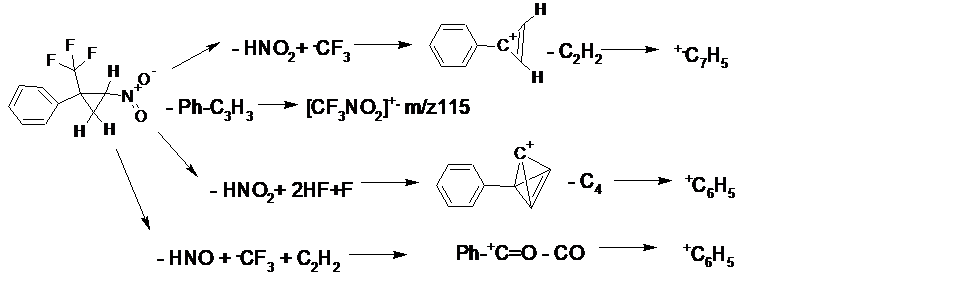
Scheme 1. Fragmentation pathways of 2-aryl-2-trifluoromethyl-1-nitrocyclopropanes.
The main pathways for fragmentation of [2-nitro-1- (trifluoromethyl) cyclopropyl] benzene are shown in Fig. 6.
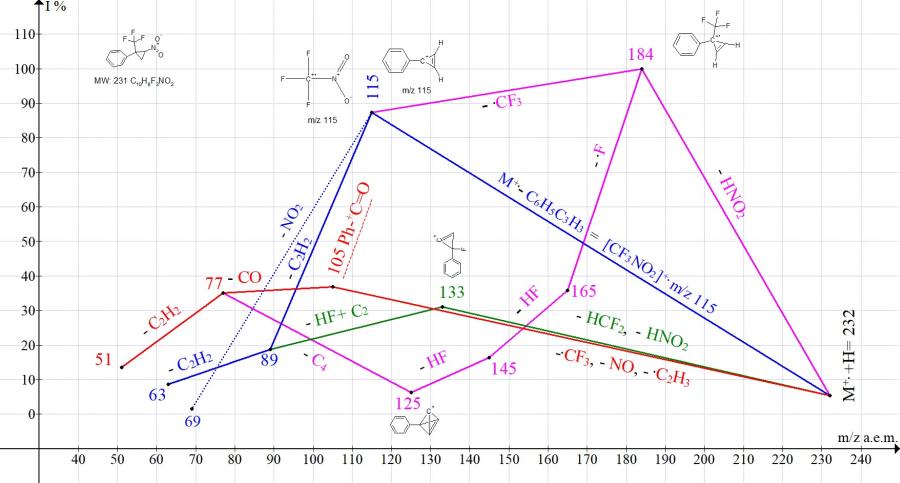
Figure 6. Mass spectrum ion series of [2-nitro-1-(trifluoromethyl)cyclopropyl]benzene C10H8F3NO2 MW: 231.
When M+. –HNO2 is ejected (Fig. 6), a basic ion peak with m/z 184 is formed. It is likely that the C3-cycle is also retained for the ion with m/z 115 after the detachment of .CF3. The ion peak with m/z 115 (87.4%) is the total peak of two ions: Ph-+C3H2 and F3C.+NO2. This fact confirms the preservation of the cyclopropene cycle both in the formed ion and in the detached neutral molecule. Fragmentation of the ion with m/z 115 (detachment of C2H2) is consistent with the structure of Ph-+C3H2.
Compared with the spectra of other derivatives of 2-aryl-2-trifluoromethyl-1-nitrocyclopropanes, in the spectrum of [2-nitro-1-(trifluoromethyl)cyclopropyl]benzene (Fig. 6) the peak intensity with m/z 105 Ph-+C=O, formed during the decomposition of cyclopropane, is minimal (36.8%). Apparently, the unsubstituted phenyl group, in comparison with substituted phenyl groups, is capable of maximally stabilizing cyclopropane.
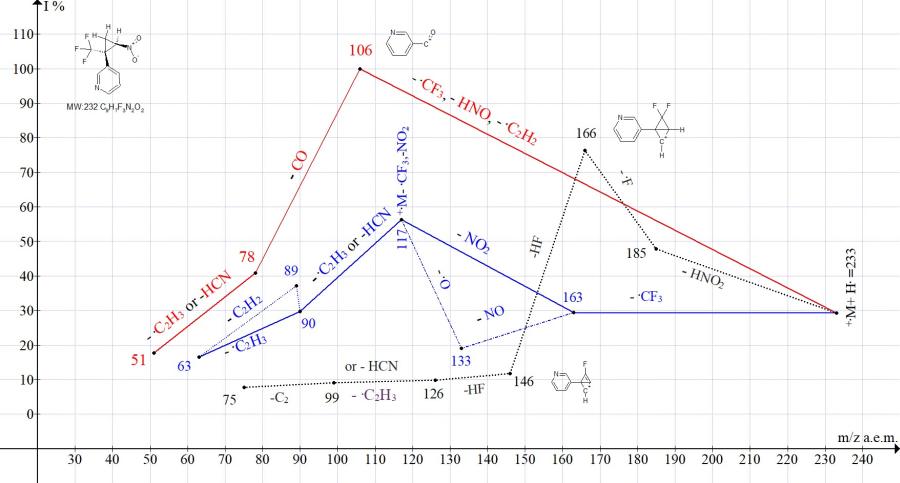
Figure 7. Mass spectrum ion series of 3-[2-nitro-1-(trifluoromethyl)cyclopropyl]pyridine C9H7F3N2O2 MW: 232.
In the spectrum of 3-[2-nitro-1-(trifluoromethyl)cyclopropyl]pyridine (Fig. 7) the peak of the radical cation [CF3NO2]+. with m/z 115 is absent. Instead, a cyclopropene peak with m/z 117 (M+. -115) with an average intensity of 56.3% is formed. The base peak of the spectrum C5NH4+C=O with m / z 106 is the result of complete decomposition of cyclopropane.
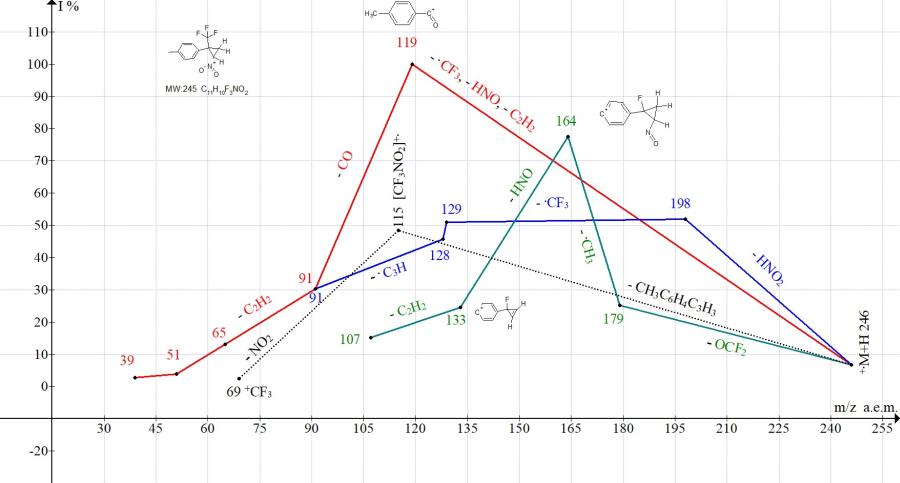
Figure 8. Mass spectrum ion series of 1-methyl-4-[2-nitro-1-(trifluoromethyl)cyclopropyl]benzene C11H10F3NO2 MW: 245.
In the mass spectrum of 1-methyl-4-[2-nitro-1-(trifluoromethyl)-cyclopropyl]benzene (Fig. 8), the peak intensity of the cyclopropene ion with m/z 129 does not exceed (51%), while the decay of the cycle leads to base peak with m/z 119 (100%). The peak intensity at m/z 115 is only 48%. That is, in comparison with [2-nitro-1-(trifluoromethyl)cyclopropyl] benzene, the methyl substituent reduces the stabilization of cyclopropene and promotes the decomposition of the cycle (Fig. 8).
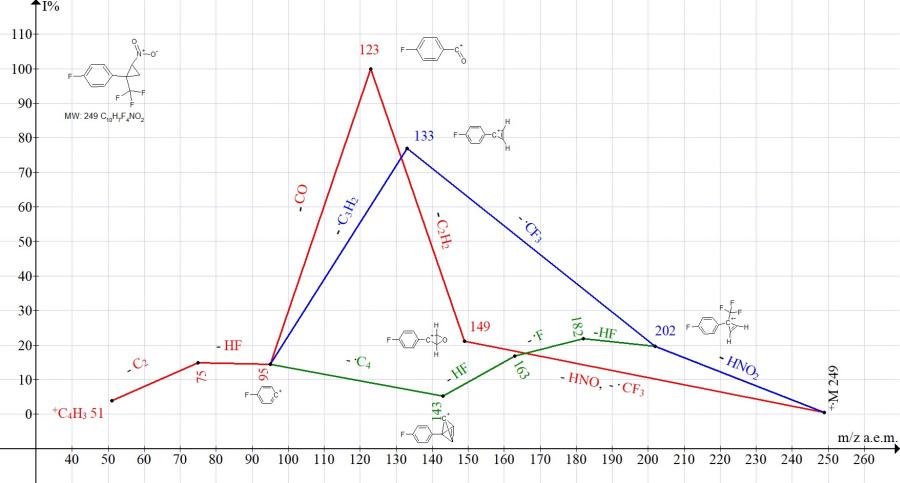
Figure 9. Mass spectrum ion series of 1-fluoro-4-[2-nitro-1-(trifluoromethyl)cyclopropyl]benzene C10H7F4NO2 MW: 249.
In the spectrum of 1-fluoro-4-[2-nitro-1-(trifluoromethyl) cyclopropyl] benzene, the ion with m/z 115 is not formed (Fig. 9). The two main fragmentation pathways are: complete decomposition of the cycle with the formation of a base ion with m/z 123, as well as detachments of HNO2 and .CF3 (M-116), leading to the peak of cyclopropene with m/z 133 (77%) (Fig. 9).
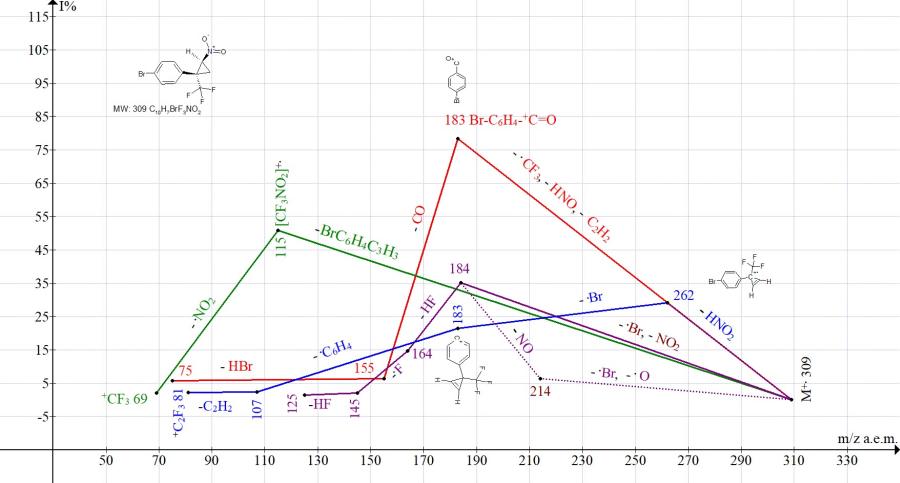
Figure 10. Mass spectrum ion series of 1-bromo-4-[2-nitro-1-(trifluoromethyl)cyclopropyl]-benzene C10H7BrF3NO2 MW: 309.
In the mass spectrum of 1-bromo-4-[2-nitro-1-(trifluoromethyl-cyclopropyl]-benzene (Fig. 10) compared with the spectrum of [2-nitro-1-(trifluoromethyl)cyclopropyl]benzene (Fig. 6) the peak intensity of the [CF3NO2]+. with m/z 115, corresponding to the retention of cyclopropane in the detached neutral molecule, decreases from 87% to 51%, and the detachment of M-116 (HNO2 + .CF3) = 193 does not occur. In accordance with the peak intensities of the isotopic ions 79 and 81, the peak with m/z 183 (Fig. 10.) belongs to two ions: by (78.4%) to the BrC6H4+C=O ion and (21.6%) to the ion +C6H4(CF3)C3H2. The peak intensity with m/z 262 (M+.-HNO2) is only 29%.
Compared with the spectrum of [2-nitro-1-(trifluoromethyl)cyclopropyl]benzene (Fig. 6), the substitution of bromine for the phenyl group does not contribute to the stabilization of cyclopropane. Peaks appear in the mass spectrum (Fig. 10), confirming the detachment of the bromine atom.
Influence of para- and meta- substituents Cl, NO2 and CF3 of the phenyl group on fragmentation of 2-aryl-2-trifluoromethyl-1-nitrocyclopropanes
Comparison of the ionic series in the mass spectra of isomers with para- and meta-substituents (Cl, NO2, and CF3) of the phenyl group confirms the influence of the position of the substituent on the fragmentation pathway and the peak intensity corresponding to decomposition or intermediate retention of the ring. Thus, in the spectrum of cyclopropane with a para-chlorophenyl group, the main fragmentation pathway is its decomposition with the formation of a base ion with m/z 139 Cl-C6H4+C=O (Fig. 11). In the spectrum of cyclopropane with a meta-chlorophenyl group, the peak intensity with m/z 139 decreased to 40%, and the base peak was the peak of cyclopropene with m/z 218 (Fig. 12). The ejection of a cyclopropene molecule with the formation of a trifluoronitromethane ion m/z 115 in isomers with a para-chlorine (25%) and meta-chlorophenyl group (31%) occurred with similar intensities.
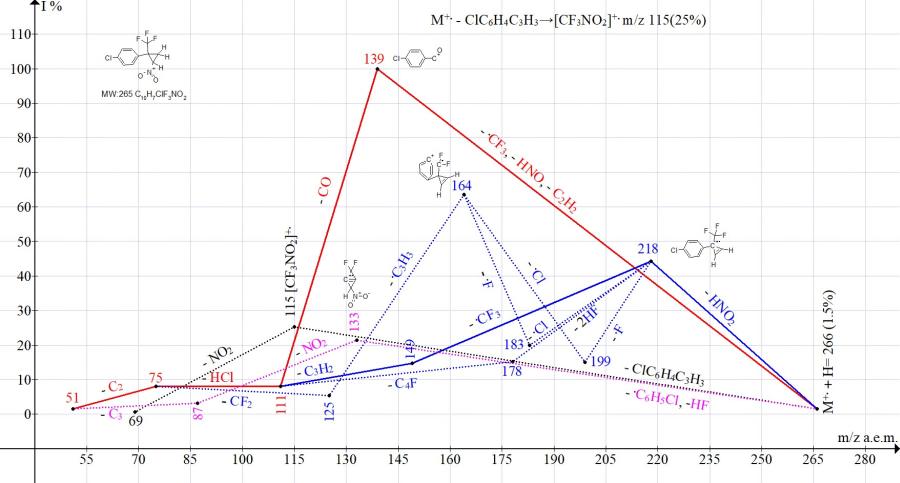
Figure 11. Mass spectrum ion series of 1-chloro-4-[2-nitro-1-(trifluoromethyl)cyclopropyl]benzene
C10H7ClF3NO2 MW: 265.
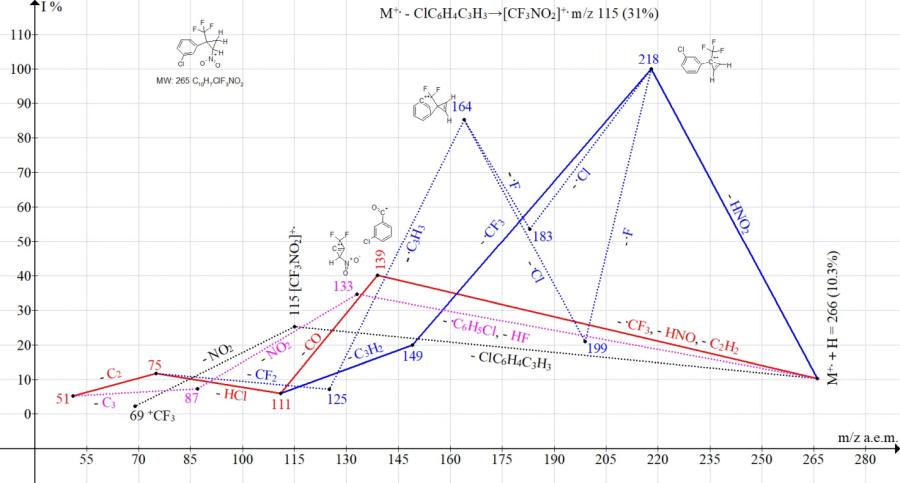
Figure 12. Mass spectrum ion series of 1-chloro-3-[2-nitro-1-(trifluoromethyl)cyclopropyl]benzene C12H7ClF3NO2 MW: 265.
Under fragmentation conditions, the isomer with the meta-chlorophenyl group (Fig. 11) demonstrates a greater ability to preserve the cycle than the isomer with the para-chlorophenyl group (Fig. 12).
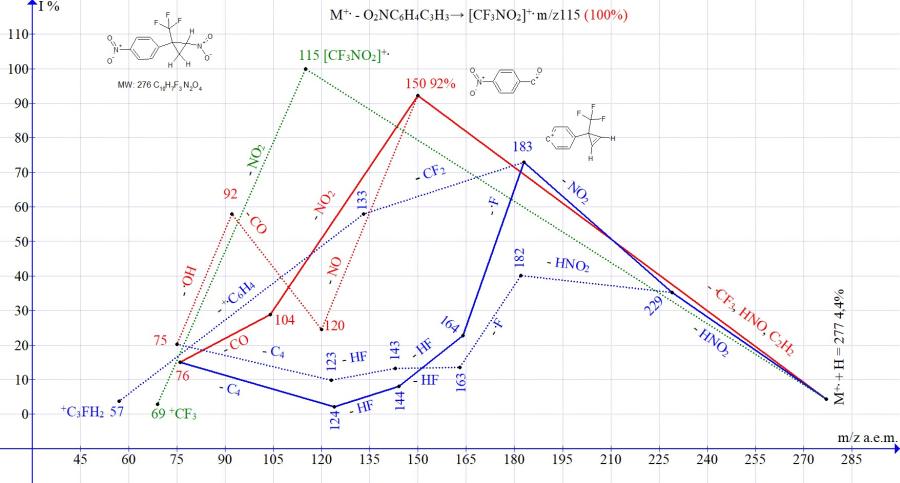
Figure 13. Mass spectrum ion series of 1-nitro-4-[2-nitro-1-(trifluoromethyl)cyclopropyl]benzene C10H7F3N2O4 MW: 276.
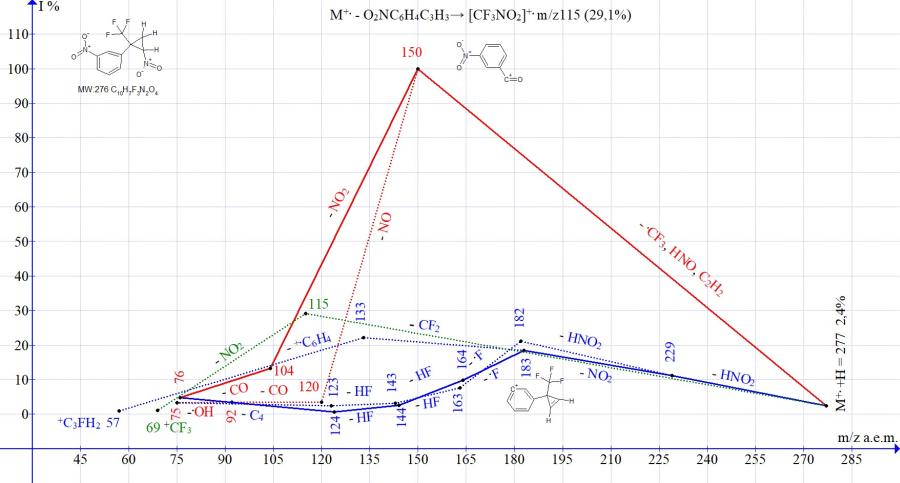
Figure 14. Mass spectrum ion series of 1-nitro-3-[2-nitro-1-(trifluoromethyl) cyclopropyl]benzene C10H7F3N2O4 MW: 276.
In the mass spectra of isomeric cyclopropanes with para- and meta-nitro-substituted phenyl groups (Figs. 13-14), the peak intensities of the ion with m/z 150, formed as a result of the complete decomposition of cyclopropane, are close (92 and 100%). The peak intensity corresponding to the ejection of the cyclopropene molecule with the formation of the trifluoronitromethane ion m/z 115 is 100% for the isomer with the para-nitrophenyl group (Fig. 13) and 29% for the isomer with the meta-nitrophenyl group (Fig. 14).
The difference in the fragmentation of isomers also manifests itself in the efficiency of double detachment of HNO2. After the detachment of HNO2, there is no detachment of .CF3, as in all spectra of nitrocyclopropanes, which do not contain the second nitro group, but a repeated emission of HNO2 or NO2. The detachments of HNO2 and NO2 with retention of the cyclopropene cycle and the formation of an ion with m/z 183 occur more efficiently (73%) in the spectrum with a para-nitrophenyl group and less efficiently (18%) in the spectrum with a meta-nitrophenyl group. Under fragmentation conditions, the isomer with the para-NO2 group shows a greater ability to maintain the cycle than the isomer with the meta-NO2 group.
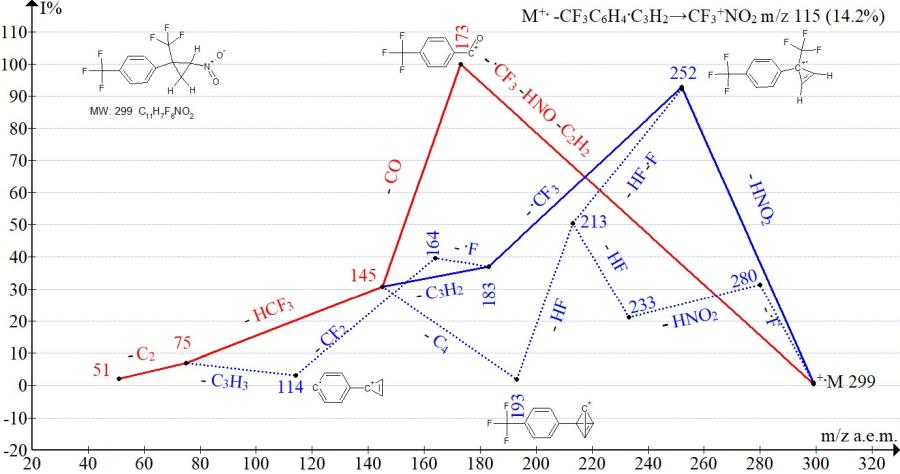
Figure 15. Mass spectrum ion series of 1-trifluoromethyl-4-[2-nitro-1-(trifluoromethyl)cyclopropyl] benzene C11H7F6NO2 MW: 299.
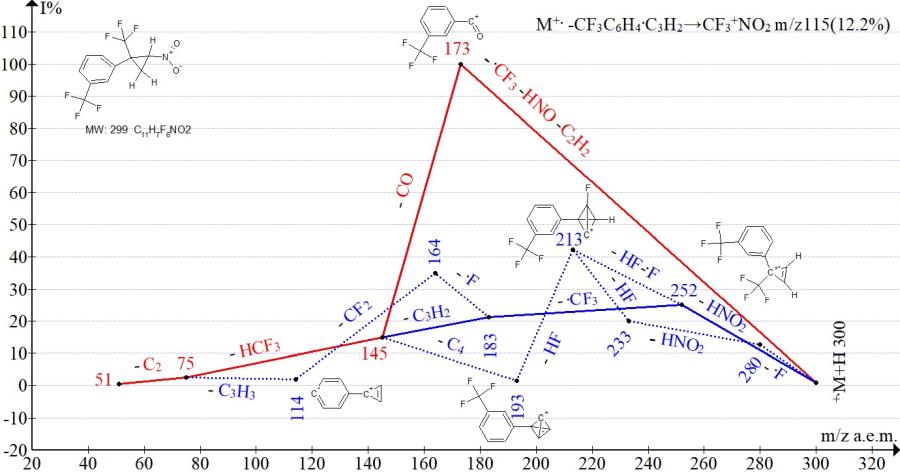
Figure 16. Mass spectrum ion series of 1-trifluoromethyl-3-[2-nitro-1-(trifluoromethyl)cyclopropyl]benzene C11H7F6NO2 MW: 299.
In the spectra of isomers with para- and meta-trifluoromethylphenyl groups (Figs. 15-16), the peak intensities of the trifluoronitromethane ion with m/z 115 are equal (14% and 12%). The peak intensities of the ion with m/z 173, corresponding to the complete decomposition of cyclopropane, are the same for the isomers (100%). The difference in fragmentation manifests itself only in the efficiency of detachment of the HNO2 group. The detachment of HNO2 with intermediate retention of the cyclopropene ring and the formation of an ion with m/z 252 more efficiently (93%) occurs in the spectrum of the isomer with the para-CF3 group and less effectively 25% in the spectrum with the meta-CF3 group.
Conclusion
Using the examples of mass spectra of compounds that do not contain regular fragment groups, the possibility of determining their ionic series corresponding to variants and sequences of competing fragmentation processes is shown. The determination of the series of spectra included consideration of the general fragmentation pathways characteristic of this class of compounds, as well as all variants of the detachment of primary radicals and their coordination with each other.
In the spectra of Cl and Br isotopic atoms, the compositions of the fragment and base ions of the series were confirmed by the corresponding peaks of isotopic ions. Ionic series in mass spectra make it possible to determine the main and secondary competitive fragmentation pathways, as well as their dependence on substituents.
Acknowledgments
This work was financially supported by the Ministry of Science and Higher Education of Russian Federation using scientific equipment of the Center of Molecules Structure Study of INEOS RAS.
References
- Kagramanov N.D., Series of fragment ions of cycloalkanes, perfluorocyclohexane, perfluoropolycycloalkanes, Fluorine notes, 2021, 3(136), 3-4.
- Sigan A.L., Golubev A.S., Belyaeva E.V., Gorfinkel S.M., Kagramanov N.D., Spiridonov Yu.Ya., Chkanikov N.D., Synthesis of ethyl 5-aryl-5-trifluoromethyl-4,5-dihydroisoxazole-3-carboxylates with growth regulating properties plants, Izv. AN, Ser. khim, 2019, 1, 99-103. (in Russian)
- Golubev A. S., Shidlovskii A. F., Peregudov A. S., Kagramanov N. D., Synthesis of 3‑fluoromethyl-2,1-benzisoxazoles, Russ. Chem. Bull., 2014, 63(10), 2264-2270.
- Patent RU2699654 (2019), Е-2-aryl-2-trifluoromethyl -1-nitrocyclopropanes and method for their production, Golubev A.S., Gorfinkel S.M., Markova A.A., Suponitsky K.Yu., Peregudov A.S., Strelkova T.V., Ostapchuk P.N., Kagramanov N.D., Chkanikov N.D.
- Ferguson L.N., Ring strain and reactivity of alicycles, J. Chem. Educ., 1970, 47, 46-53..
- Chochua K.A., Chizhov O.S., Shabarov Yu.S., Kazbulatova N.A., Mass spectrometric study of cyclopropane derivatives. I. Hydrocarbons of the phenylcyclopropane series and related compounds, Russian Journal of Organic Chemistry, 1971, 7, 2024-2028. (in Russian)
- Zaikin V.G., Mikaya A.I., Vdovin V.M., Small cycle mass spectrometry., Moscow, Nauka, 1983. (in Russian)
- Averina E.B., Yashin N.V., Kuznetsova T.S., Zefirov N.S., Uspekhi khimii, 2009, 78(10), 963-979. (in Russian)
ARTICLE INFO
Received 22 September 2021
Accepted 28 September 2021
Available online October 2021
Recommended for publication by PhD M. A. Manaenkova
Fluorine Notes, 2021, 138, 5-6
