Received: March 2022
DOI 10.17677/fn20714807.2022.02.02
Fluorine Notes, 2022, 141, 3-4
ACTIVATION ENERGY AND THERMAL EFFECT OF INITIATION REACTION OF CATIONIC ISOBUTYLENE POLYMERIZATION IN THE PRESENCE OF COMPLEX CATALYST «BORON FLUORIDE – WATER» IN HEPTANE
V.A. Babkin1, D. S. Andreev1, A. V. Ignatov1, E. S. Titov2,3, A.I. Rakhimov2, N.A. Schreibert2
1Volgograd State Technical University (Sebryakovsky br.), 403343 Volgograd Region, Mikhailovka, Michurina st., 21.
e-mail:
babkin_v.a@mail.ru
2Volgograd State Technical University,400005 Volgograd, Lenin av., 28.
e-mail:
organic@vstu.ru
3Volgograd State Medical University,400131 Pavshikh Bortsov sq., 1.
e-mail: titova051@rambler.ru
Abstract: This paper presents data on activation energy and thermal effects at the stage of initiation of cationic isobutylene polymerization in the presence of complex catalyst boron fluoride – water in heptane with various stoichiometric composition, obtained by quantum chemical calculation using ab initio method. It has been established that increase in heptane amount leads to decrease in activation energy of reaction studied.
Keywords: isobutylene, boron fluoride-water catalyst, activation energy, reaction heat, solvent, heptane, ab initio method.
Introduction
Heptane is a classical solvent widely used in various chemical reactions and, in particular, in cationic polymerization of various olefins [1]. Often (especially when modeling the mechanisms of various chemical reactions) the influence of solvents was practically not taken into account and equated to the same reactions, but which run in vacuum or in absence of solvent [2, 3]. Nevertheless, it has already been noted that reaction rates (even when using indifferent solvents [4]) differ from the rates that occur in absence of solvents. That is, the solvent, even if it is indifferent, can significantly affect the activation energies of reactions studied [4].
In this regard, the following tasks were set: to clarify the effect of heptane solvent amount on mechanism and energy of initiation reaction of isobutylene cationic polymerization (thermal effect EA and activation energy Q). For this, the system BF3-H2O - isobutylene - heptane (II) with following stoichiometric composition was studied: BF3-H2O – 1 (BF3-H2O):1 (heptane), 1:2, 1:3, 1:4.
It is advisable to estimate the values of activation barriers and thermal effects theoretically using quantum chemical calculation of studied initiation mechanisms of cationic isobutylene polymerization due to the fact that it is very difficult (and often impossible) to estimate the activation energy by experimental methods.
Methodical part
To study the mechanism of cationic polymerization initiation of isobutylene in the presence of above complex catalyst, we chose the classical ab initio quantum chemical method in 6-311G** basis [5–6], which very accurately estimates the activation energies and thermal effects of studied reactions [7–8]. The reaction was modeled using MacMolPlt program [9].
This initiation mechanism was studied along one coordinate (similarly to [10–12]). For process studied as reaction coordinate the RC(1)-H(13) bond and multiplicity M=1 was chosen, since M=2S+1 (where S - the total spin is 0 due to the fact that all electrons are paired). Throughout the interaction between catalyst and isobutylene, at each step the charge conservation law was clearly observed; that is, the distributed total charge at all atoms was equal to zero. The simulation was performed in accordance with Markovnikov's rule, when the proton attacks the most hydrogenated carbon atom of p-methylstyrene - Cα(1).
Calculation results
The initial models of initiation reaction of cationic isobutylene polymerization in the presence of BF3-H2O complex catalyst (without solvent) and in heptane with stoichiometric composition of 1:1 are shown in Figs. 1 and 2, and final structures after interaction of catalyst with monomer are shown in Figs. 3 and 4. Changes in total energy of system along reaction coordinate R without solvent and stoichiometric composition 1:1, 1:2, 1:3, 1:4 are shown in Figs. 5 and 6. The values of activation energies for different stoichiometric composition of components are presented in Table 1.
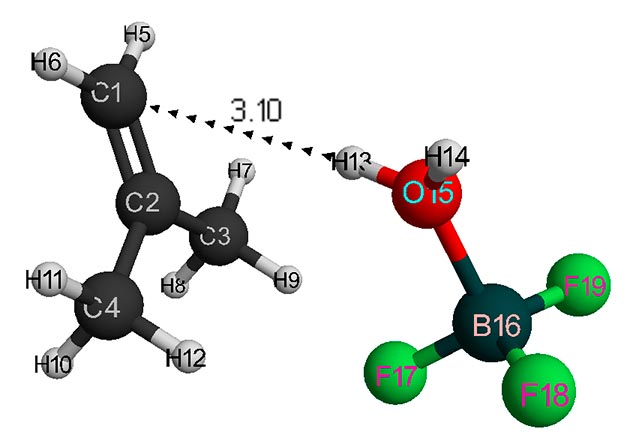
Figure 1. Structure of initial interaction model of isobutylene with a complex catalyst H2O·BF3 (without solvent).
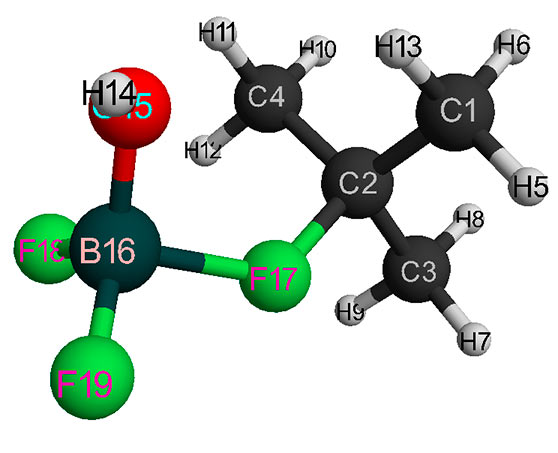
Figure 2. Final interaction structure of isobutylene with complex catalyst H2O·BF3 (without solvent).
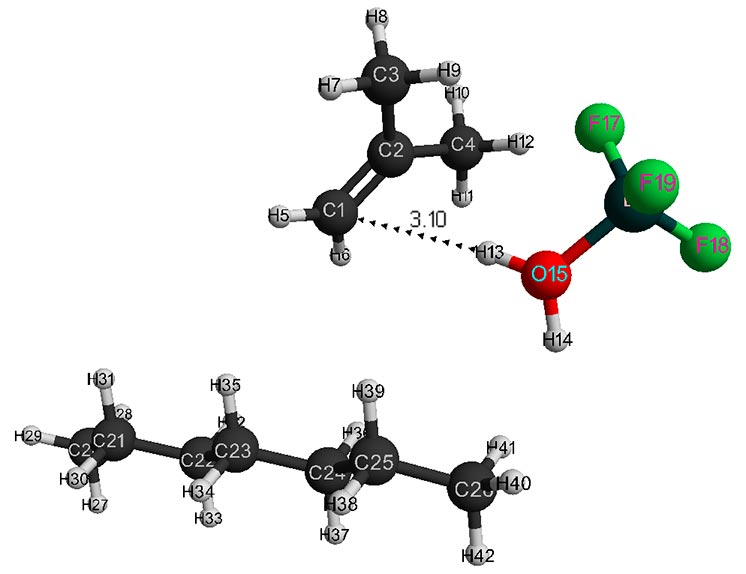
Figure 3. Initial model structure of isobutylene interaction with complex catalyst H2O·BF3 - 1 heptane.
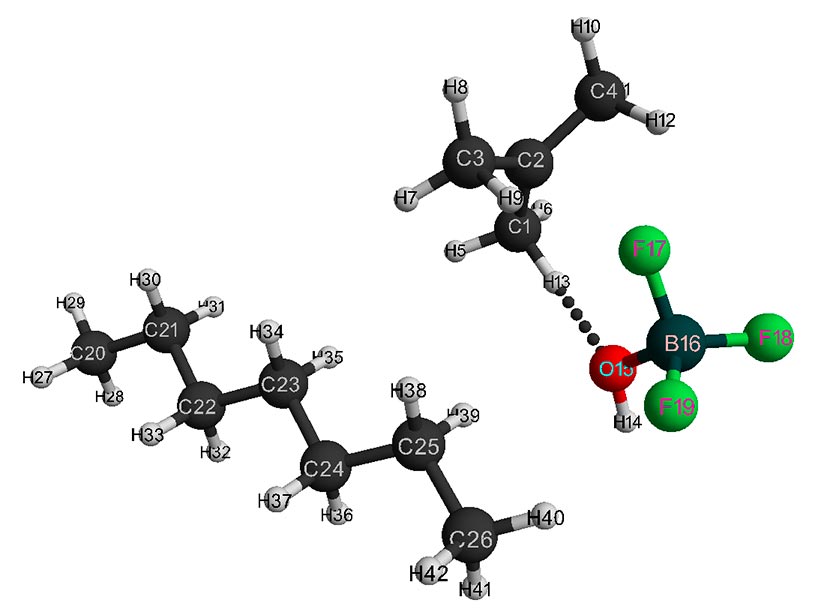
Figure 4. Final interaction structure of isobutylene with complex catalyst H2O·BF3 - 1 heptane.
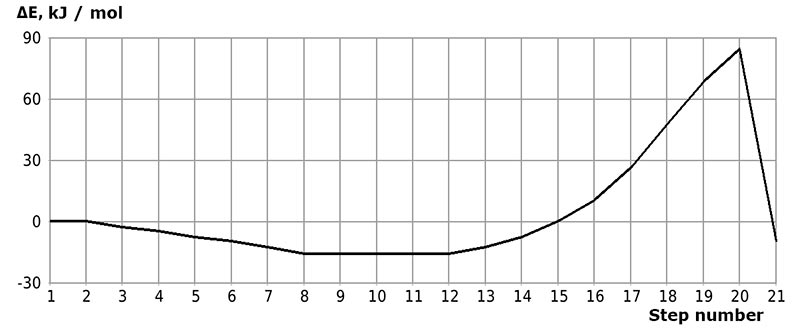
Figure 5. Change in total energy (ΔE) of interaction reaction of isobutylene with complex catalyst H2O·BF3 without solvent (# 1-21 of interaction steps).
Table 1. Activation energies (ЕA) and thermal effects (Q) of reaction of isobutylene with complex catalyst H2O·BF3 with different stoichiometric composition.
|
Item |
Composition of molecular system |
ЕA, kJ/mol |
Q, kJ/mol |
|
1. |
Isobutylene - BF3 H2O (without heptane) |
100 |
-6 |
|
2. |
Isobutylene - BF3 H2O – 1 heptane (1:1) |
95 |
-95 |
|
3. |
Isobutylene - BF3 H2O – 2 heptane (1:2) |
95 |
-11 |
|
4. |
Isobutylene - BF3 H2O – 3 heptane (1:3) |
92 |
-92 |
|
5. |
Isobutylene - BF3 H2O – 4 heptane (1:4) |
87 |
-76 |
Behavior fragments analysis of complex catalyst BF3-H2O and isobutylene (breaking of O-H bond and converting π-bond of monomer into σ-bond, as well as the formation of new O-C and C-H bonds, the change in total energy along reaction coordinate R (see Fig. 5- 6) shows that this initiation mechanism is a coordinated process with simultaneous breaking and formation of above bonds (similar to [12]). In this case, the amount of heptane has a very significant effect on activation reaction energy (EA): as the amount of heptane increased, it decreases from 95 kJ/mol to 87 kJ/mol. These reactions are endothermic and have a barrier nature.
In is obviously, that obtained results of quantum chemical calculations should be experimentally verified.
Conclusion
Thus, for the first time we have carried out the systematic studies of initiation mechanism of cationic isobutylene polymerization in the presence of BF3-H2O complex catalyst in heptane in the systems with various stoichiometric compositions. It has been established that increase in the amount of heptane leads to decrease in activation energy.
Ultimately, the obtained dependencies, together with well-known fact - control of olefin initiation reaction by varying the nature of ligand environment of Lewis acid (BF3) and Bronsted acid (HF, H2O), offer a new opportunity to control the reaction studied by changing the stoichiometric composition of catalyst with respect to the solvent - heptane, and obtain the isobutylene polymer with predetermined properties.
References
- Kennedy, J., Cationic polymerization of olefins, «Mir» Publishing House, Moscow, 1978, 431 p. (in Russian).
- Accounting for solvent in quantum-chemical modeling of the synthesis mechanism of polyarylenephthalides, O.V. Matsevich, Bulletin of Kazan Technological University, 2014, 17 (10), 24-26. (in Russian).
- Quantum-chemical aspects of cationic polymerization of olefins, V.A. Babkin [and others], 1996, «Gilem» Publishing House (Ufa), 188 p. (in Russian).
- Babkin, V. A. [and others], Energetics of the initiation reaction of cationic polymerization of ethylene, propylene and isobutylene. The catalyst is an aquacomplex of aluminum chloride. The solvent is toluene, Volgograd: VolgGTU, 2021, 156 p. (in Russian).
- General Atomic and Molecular Electronic Structure System., M.W. Schmidt [and others], J.Comput.Chem., 1993, 14, 1347-1363.
- Granovsky, A.A., Firefly version 8, 2013. http://classic.chem.msu.su/gran/firefly/index.html
- Ermakov, A.T. Quantum mechanics and quantum chemistry. «Yurayt» Publishing House, Moscow, 2016, 555 p. (in Russian).
- Cirelson V.G., Quantum Chemistry. Molecules, molecular systems and solids, Moscow, Publishing House «Binom», 2010, 496 p. (in Russian).
- MacMolPlt: A Graphical User Interface for GAMESS., B.M. Bode, M.S. Gordon, Journal of Molecular Graphics, 1998, 16, 133-138.
- Study of interaction of complex HF·BF3 -catalyst with p-methylstyrene AB INITIO method, V. A. Babkin [and others], Fluorine Notes, 2020, 1(128), 1-2.
- Studying the mechanism of interaction of the complex catalyst aluminum chloride - hydrochloric acid and p-methylstyrene in toluene, V.A. Babkin [and others], Bulletin of Kazan Technological University, 2020, 1, 9-12.
- Quantum Chemical Calculation of Initiation Mechanism of Cationic Polymerisation of Propylene with Chloride–Aluminium Aquacomplex, V. A. Babkin [and others], Oxidation Communications, 2020, 43(1), 24-29.
ARTICLE INFO
Received 2 March 2022
Accepted 14 March 2022
Available online April 2022
Recommended for publication by PhD V. Don
eLIBRARY Document Number (EDN) IUYGVC

Fluorine Notes, 2022, 141, 3-4
