Received: April 2022
DOI 10.17677/fn20714807.2022.02.03
Fluorine Notes, 2022, 141, 5-6
EFFECT OF 2,3-BIS(POLYFLUOROALKOXY)-[1,4]-NAPHTHOQUINONES AND 2-CHLORO-3-(2-PROPENOXY)-[1,4]-NAPHTHOQUINON ON HEAT RESISTANCE OF POLY(METHYL METHACRYLATE)
O. A. Melnik, M. I. Buzin, V. I. Dyachenko
A. N. Nesmeyanov Institute of Organoelement Compounds RAS RF, 119991, Moscow, Vavilova st., 28,
e-mail: omel@ineos.ac.ru
Abstract: Copolymers of methyl methacrylate with 2-chloro-3-(2-propenoxy)-1,4-naphthoquinone and polymers with additions of 2,3-bis(polyfluoroalkoxy-[1,4]-naphthoquinones) have been synthesized. Their physicochemical properties and thermal characteristics have been studied. It has been established that using of 2,3-disubstituted fluorine-containing [1,4]-naphthoquinones as additive leads to increase in thermal-oxidative stability of poly(methyl methacrylate).
Keywords: 2-chloro-3-(2-propenoxy)-1,4-naphthoquinone, 2,3-bis(polyfluoroalkoxy)-[1,4]-naphthoquinones, poly(methyl methacrylate), free-radical (co)polymerization, heat resistance
Acrylic polymers, characterized by high transparency and good solubility, have found wide practical application for creation of various materials. Poly(methyl methacrylate) (PMMA) is a transparent polymer with a good optical, mechanical and electrical insulating properties and high weather resistance; it is used as a structural material in aircraft, automobile, instrumentation and also in shipbuilding, medicine, optics and lighting engineering [1, 2]. To reduce destructive processes at elevated temperatures, stabilizers, various modifying additives and antioxidants are introduced into these polymers [1].
Previously, we showed the stabilizing effect of 2-chloro-3-polyfluoroalkoxy-[1,4]-naphthoquinones (having different lengths of polyfluoroalkoxy radical) on PMMA thermal-oxidative stability [3]. It is known that copolymerization is often used to modify polymers in order to improve their functional (including thermal) characteristics [4]. In the present paper, the copolymers of methyl methacrylate (MMA) and 2-chloro-3-(2-propenoxy)-1,4-naphthoquinone with various compositions were synthesized by free-radical bulk copolymerization, and their heat resistance was determined.
For the first time, 2-chloro-3-(2-propenoxy)-1,4-naphthoquinone (III) was obtained by reaction of 2,3-dichloro-1,4-naphthoquinone (I) with allyl alcohol (II) in tetrahydrofuran in the presence of К2СО3 throughout 3 hours [5]. After removal of solvent in a vacuum and subsequent chromatography of residue on silica gel, the yield of compound III was 78%.
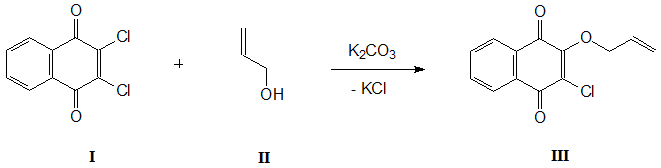
Scheme 1.
We managed to simplify the reaction conditions and procedure for isolating the final product, significantly increasing its yield. It turned out that when this reaction is carried out in allyl alcohol in the presence of equimolar amount of potash, the compound III is formed within 1.5 hours with a quantitative yield. This greatly simplifies the procedure for its isolation, which consists in diluting the reaction mass with water and filtering the precipitated reaction product. After washing it with distilled water and drying on a glass filter, the spectrally pure 2-chloro-3-(2-propenoxy)-1,4-naphthoquinone (III) is obtained.
Free-radical copolymerization of MMA and compound III (1-3 mol.%) was carried out in evacuated, sealed glass ampoules in the presence of 0.5 wt.% of initiator - azobisisobutyric acid dinitrile (AIBN) at 60°C. The copolymers are the solid transparent glassy samples of yellow color, their structure was established according to results of IR spectroscopy and elemental analysis (see Fig. 1).
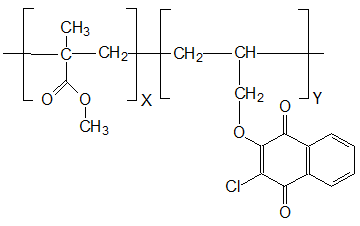
Figure 1. Structural formula of copolymers MMA and 2-chloro-3-(2-propenoxy)-1,4-naphthoquinone.
They are soluble in benzene, toluene, chlorinated hydrocarbons and insoluble in hexane, sulfuric ether, lower alcohols and water.
After studying the main physicochemical characteristics of obtained copolymers, it seemed appropriate to study the possibility of using 2,3-bis(polyfluoroalkoxy)-[1,4]-naphthoquinones (see Fig. 2) as an antioxidant additives for MMA polymerization. Previously, it was shown by cyclic voltammetry method that these compounds, like 2-chloro-3-polyfluoroalkoxy-[1,4]-naphthoquinones, easily undergo by step-by-step reversible electroreduction in an aprotic medium (reduction potential Ео1 = -0.35 V) [6]. That is, it can slow down the thermal-oxidative degradation of polymers by neutralizing of resulting radicals.
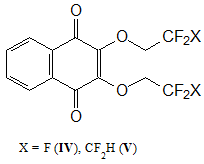
Figure 2. Structural formulas of 2,3-bis(polyfluoroalkoxy)-[1,4]-naphthoquinones.
These compounds are low-melting, stable, highly soluble substances in organic solvents. It can be obtained by reaction of commercially available 2,3-dichloro-[1,4]-naphthoquinone and commercially produced fluorine-containing alcohols [7]. Free-radical bulk MMA polymerization was used to synthesize PMMA samples with additions of compounds IV and V, and its thermal- oxidative stability was studied.
Thermal stability of obtained (co)polymers was evaluated by decomposition start temperature Тd, which was taken as a temperature at which the mass loss of analyzed sample was 10% of initial value. It was determined by dynamic thermogravimetric analysis at a heating rate of 10°C/min in air. The results are shown in the Table 1.
Comparison of obtained results with the data of thermogravimetric PMMA analysis indicates a decrease in the thermooxidative stability of copolymers MMA and 2-chloro-3-(2-propenoxy)-1,4-naphthoquinone (samples 3 and 3'). Thus, the Тd for copolymer MMA and compound III with a molar composition of 99:1 is equal to the Тd for PMMA (265°C). With increasing in the content of monomer III to 3 mol.%, Тd is 208°C. This may be due to the fact that during copolymerization of allyl compound III, the chain is transferred to the monomer, which leads to decreasing in the molecular weight and reduction in Тd [8, 9].
Table 1. Thermal stability of MMA (co)polymers with additives of 2,3-substituted [1,4]-naphthoquinones of general formula:
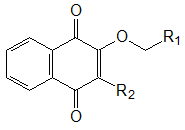
|
Sample No |
R1 |
R2 |
Тd, °C |
|
|
99:1 |
97:3 |
|||
|
PММА* |
– |
– |
265 |
|
|
1, 1’* |
CF3 |
Cl |
305 |
320 |
|
2, 2’* |
CF2CF2H |
Cl |
306 |
315 |
|
3, 3’ |
CH=CH2 |
Cl |
265 |
208 |
|
4, 4’ |
CF3 |
OCH2CF3 |
320 |
302 |
|
5, 5’ |
CF2CF2H |
OCH2C2F4H |
320 |
304 |
* data of [3]
It was found that Тd of PMMA composites with additions of 1 mol. % 2,3 bis(polyfluoroalkoxy)-[1,4]-naphthoquinones IV, V is 320°C and its value does not depend on the length of polyfluoroalkyl substituent (samples 4 and 5) . The MMA polymer with addition of 3 mol % 2-chloro-3-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (sample 1') had the same Тd value.
Experimental part
1Н and 19F NMR spectra were recorded in CDCl3 via Bruker Avance 400 spectrograph (at 400 and 376 MHz, respectively). Chemical shift 1H are given relative to Me4Si (as an internal standard), and chemical shift 19F - relative to CF3CO2H (for compound IV) and СFCl3 (for compound V) – as an external standard. IR spectra were taken via Bruker Vertex 70 v Fourier spectrometer having resolution of 4 cm-1 in attenuated total reflectance infra-red spectra mode using a PIKE Glady ATR attachment with a diamond working element. Dynamic thermogravimetric analysis was carried out via MOM Q-1500 derivatograph.
The starting reagents were 2,3-dichloro-[1,4]-naphthoquinone, 98% [CAS 117-80-6] and allyl alcohol, 99.5% [CAS 107-18-6] from ABCR company. MMA (Aldrich, 99%) was distilled under reduced pressure. AIBN (Aldrich, 98%) was purified by recrystallization from methanol.
2-Chloro-3-(2-propenoxy)-1,4-naphthoquinone (III)
227 mg (1 mmol) of 2,3-dichloro-[1,4]-naphthoquinone, 1.5 g of allyl alcohol and 138 mg (1 mmol) of anhydrous potash were placed in a glass conical flask equipped with magnetic stirrer and reflux condenser with calcium chloride tube. The reaction was carried out with stirring at room temperature for 1.5 hours. TLC control of this reaction was carried out in the system chloroform-cyclohexane = 2:3. With vigorous stirring, 5 ml of distilled water was added dropwise to the reaction mass. The resulting yellow precipitate was filtered off, washed with water and dried on the filter to constant weight. Obtaining 235 mg of light yellow amorphous compound. Yield 98.7%, m.p. 75-76С. Founded, %: C, 62.58; H, 3.71; Cl, 14.31. C13H9ClO3. Calculated, %: C, 62.70; H, 3.65; Cl, 14.24. 1Н NMR (400 MHz, СDСl3, δ, ppm, J/Hz): 5.10 (br.s, 2H, OCH2), 5.31 (br.s, 1H, CH=CH2, 3J = 10.8 Hz), 5.45 (br.d, 1H, CH=CH2, 3J = 17.4 Hz), 6.02–6.10 (m, 1H, CH=CH2), 7.75 (br.s, 2H, Ar), 8.08 (br.d, 1H, Ar, 3J = 7.8 Hz), 8.14 (br.d, 1H, Ar, 3J = 7.8 Hz).
2,3-Bis(polyfluoroalkoxy)-[1,4]-naphthoquinones were synthesized according to the procedure described in [7].
2,3-Bis-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (IV)
M. p. 81-82С. Founded, %: C, 47.58; H, 2.24; F, 31.88. C14H8F6O4. Calculated, %: C, 47.47; H, 2.28; F, 32.18. 1H NMR (CDCl3, δ, ppm, J/Hz): 8.10 (dq, 2H, Ar); 7.78 (dq, 2H, Ar), ABCD system; 4.78 (q, 4H, 2OCH2, 2J=8). 19F NMR (CDCl3, δ, ppm, J/Hz): 2.77 (s, 3F, CF3).
2,3-Bis-(2,2,3,3-tetrafluoropropoxy)-[1,4]-naphthoquinone (V)
M.p. 42-43С. Founded, %: C, 45.78; H, 2.25; F, 36.02. C16H10F8O4. Calculated, %: С, 45.95; H, 2.41; F, 36.34. 1H NMR (CDCl3, δ, ppm, J/Hz): 8.09 (dq, 2H, Ar); 7.79 (dq, 2H, Ar), ABCD system; 6.15 (tt, 2H, 2CF2H, 2JH-F=64, 3JH-F =4); 4.78 (t, 4H, OCH2, 2JH-F =16). 19F NMR (CDCl3, δ, ppm, J/Hz): -125.40 (s, 2F, CF2); -138.86 (s, 2F, CF2).
Preparation of copolymer MMA and 2-chloro-3-(2-propenoxy)-1,4-naphthoquinone (III) at a molar ratio of chains 99:1
To a mixture of 2.640 g of freshly distilled MMA and 0.066 g (III) was added 0.014 g (0.5 wt. %) AIBN as a copolymerization initiator. The reaction mixture was filtered into a glass ampoule and freed from air by three repetitions of freeze/thaw cycles in a vacuum. After that, the ampoule was sealed and placed in a thermostat heated to 60°C. After 6 hours, the ampoule was removed, cooled and opened. The clear yellow solid copolymer was dried in a vacuum at 40°C for 24 hours to constant weight. Founded, %: C, 60.29; H, 7.88. Calculated, %: C, 60.12; H, 7.97. IR spectrum of copolymer contains absorption bands characteristic of both MMA and compound III chains: 1674, 1590, 1568 cm–1 ([1,4]-naphthoquinone fragments); 1721 cm-1 (C=O MMA). There are no absorption bands of stretching vibrations of C=C bonds at 1622 and 1640 cm-1, which were present in IR spectra of initial monomers.
Copolymers of MMA and compound III were synthesized similarly at a molar ratio of chains 97:3.
Preparation PMMA with 2,3-bis-(2,2,2-trifluoroethoxy)-[1,4]-naphthoquinone (IV) at a molar ratio of 99:1
To a solution of 2.473 g of freshly distilled methyl methacrylate and 0.089 g (IV) was added 0.013 g (0.5 wt.%) AIBN as a polymerization initiator. The prepared reaction mass was filtered into a glass ampoule, which was then degassed by freezing three times by immersion in liquid nitrogen followed by thawing in a vacuum, sealed and placed in the thermostat. Polymerization temperature was 60°C. After 6 hours, the ampoule was removed, cooled and opened. The clear yellow solid polymer was dried in a vacuum at 40°C for 24 hours to constant weight.
Preparation PMMA with 2,3-bis-(2,2,3,3-tetrafluoropropoxy)-[1,4]-naphthoquinone (V) at a molar ratio of 99:1
Prepared similarly to above method from 2.425 g of freshly distilled MMA and 0.313 g of compound V.
The same method was used to synthesize PMMA samples with compounds IV, V at a molar ratio of 97:3.
Summary
Thus, the relationship between the structure of obtained 2,3-disubstituted [1,4]-naphthoquinones and the possibility of their use as antioxidant additives in MMA polymerization has been established. It is shown that using of 1 mol.% 2,3-bis(polyfluoroalkoxy)-[1,4]-naphthoquinones IV, V leads to increasing in thermal-oxidative stability of PMMA by 55°C.
Acknowledgement
This study was supported by Ministry of Science and Higher Education of the Russian Federation using the scientific equipment of the Center for Research on Structure of Molecules of INEOS RAS.
References
- Technical properties of polymeric materials, Kryzhanovsky V.C. ed., 2nd ed. S-Pb., Professiya, 2005, 235 pp. (in Russian)
- Debsky V., Poly(methyl methacrylate), Moscow, Khimiya, 1972, 152 pp. (in Russian)
- Melnik O.A., Buzin M.I., Dyachenko V.I., Fluorine notes, 2021, 138(5), 1-2.
- Kireev V.V., High molecular weight compounds, Moscow, Vysshaya shkola, 1992, 512 pp. (in Russian)
- Kadela-Tomanek M., Bebenek E., Chrobak E., Latocha M., Boryczka S., Molecules, 2017, 22, 447.
- Peregudova S.M., Dyachenko V.I., Fluorine notes, 2021, 135(2), 7-8
- Dyachenko V.I., Fluorine notes, 2021, 134(1), 5-6
- G. Moad, D. H. Solomon. The сhemistry of radical polymerization, 2nd ed. Amsterdam, Elsevier, 2006, p. 296.
- A. Matsumoto, T. Kumagai, H. Aota, H. Kawasaki, R. Arakawa, Polymer Journal, 2009, 41(1), 26–33.
ARTICLE INFO
Received 7 April 2022
Accepted 18 April 2022
Available online April 2022
Recommended for publication by PhD M. Manaenkova
eLIBRARY Document Number (EDN) LBALED

Fluorine Notes, 2022, 141, 5-6
