Received: July 2022
DOI 10.17677/fn20714807.2022.05.01
Fluorine Notes, 2022, 144, 1-2
DECAY SEQUENCES - ION SERIES OF MASS SPECTRA HEXAMETHYLBENZENE AND HEXAKIS(TRIFLUOROMETHYL)BENZENE
N. D. Kagramanov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences,
119991, GSP-1, Moscow, B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: This report on the results of the analysis of decay sequences - ion series
of mass spectra: hexamethylbenzene and hexakis(trifluoromethyl)-benzene, is a continuation of the
article on the ion series of benzene and hexafluorobenzene. In contrast to the six ion series of
the mass spectrum of benzene, the mass spectrum of hexamethylbenzene consists of eleven series of
ions. The first three ionic series of the mass spectrum of hexamethylbenzene +.M1-3,
including the elimination of groups: (.CH3+6 .H),
(C2H4 + 7.H) and (C3H6 +
5.H) with the formation of ions +С11H9 , +С10H7 and +C9H7 - the result
of agmentation of methyl substituents that occur without rearrangement of the existing π-conjugations
of the six-membered ring.
The four final series of +.M8-11 ions are formed upon
detachment of neutral molecules C8H11 and C9H13, as well
as radicals .C10H13 and .C11H15 , in which the 6-membered ring is also retained. As a result of four series +.M8-11,
ions +C4H7 m/z 55, +C3H5 m/z 41,
+C2H5 m/z 29 and +CH3 m/z 15 appear.
Two intermediate series of +.M4-5 hexamethylbenzene
ions are formed upon detachments: dimethylacetylene CH3CCCH3 (-C4H6)
conjugation π1-4, and also dimethylacetylene with an additional, synchronous detachment
.CH3 (+.M - .C5H9).
Two more series of +.M6-7 ions are the decay of +.M/2
(emission of the .C6H9 radical), the conjugation
of π1-3, and the detachment of the rearrangement radical *.С7H11 (-M/2
+ CH2). Thus, in four +.M4-7 fragment series, two
pairs of ions are formed: +C8H12, *+C7H9 with π1-4 conjugation and +C6H9 , *+C5H7 with π1-3 conjugation.
Compared with the mass spectrum of benzene and five variants of its rearrangement π‑conjugations, only
two variants of rearrangement π1-4 and π1-3 are realized in the spectrum of
hexamethylbenzene.
Unlike the two branched ion series of the mass spectrum of hexafluorobenzene, the mass spectrum of hexakis(trifluoromethyl)benzene
consists of five series of ions, with two series also branching.
the emission of one fluorine atom to form the ion +C12F17 m/z 467(87%)
and the detachment of CF2 to form the base ion +C11F15 m/z417. The next five CF2 emissions end with the +C6F5 m/z167 ion (1.8%). In this series, the π-conjugations of the original 6-term cycle are preserved.
Two more ion series arise after the simultaneous detachment of two and also three fluorine atoms and
subsequent CF2 emissions. The fourth series of +.M4 ions is the result of the ejection of the rearrangement perfluoroallyl radical *CF2=CF-.CF2
m/z 131. The fifth series, consisting of two ions, includes the detachment of the .C11F15 radical, which retains the π-conjugations of the 6-membered cycle. As a result, the +CF3 ion m/z 69(66%) appears.
Keywords: ionic series of mass spectra, regular fragment groups (СCH3)6, (СCF3)6, examethylbenzene, hexakis(trifluoromethyl)benzene.
Introduction
Decay sequences - ion series of mass spectra of benzene and hexafluorobenzene, as well as its isomers were presented earlier [1]. In contrast to the five rearrangements of π-conjugations of carbon atoms of benzene, no rearrangements of π-conjugations of the ring occur in the spectrum of hexafluorobenzene and its isomers. And the number of series of ions is reduced to two. The reasons for the impossibility of rearrangement of π-conjugations +.M of hexafluorobenzene are the greater strength of π-bonds of fluorine-substituted carbon atoms of the cycle, compared with the bonds of hydrogen-substituted carbon atoms of benzene, as well as the difference masses of substituents - hydrogen and fluorine atoms.
Since both ionic series of the mass spectrum of hexafluorobenzene [1] exclude the rearrangement of its π-conjugations, it should not be expected in the case of hexakis(trifluoromethyl)-benzene either. The spectra of hexamethylbenzene and hexakis(trifluoromethyl)benzene are presented in the WILEY and NIST mass spectrometric libraries, but they are not described in the literature.
X-ray diffraction analysis of hexamethylbenzene crystals was first performed by Kathleen Lonsdale in 1927. He found that the hexamethylbenzene molecule is flat, and the distances between the carbon atoms in the ring are the same, which was an important proof of its aromaticity. The Raman scattering spectrum of hexamethylbenzene was studied in the temperature range from room temperature to 2°K [2]. Changes in the structure of hexamethylbenzene depending on temperature were also studied by powder neutron diffraction [3].
The aim of this work is to analyze the spectra and ion series of benzene and hexamethylbenzene, as well as hexafluorobenzene and hexakis(trifluoromethyl)benzene.
Ionic series of the mass spectrum of hexamethylbenzene
The mass spectrum of hexamethylbenzene (Fig.1.) consists of eleven ion series.
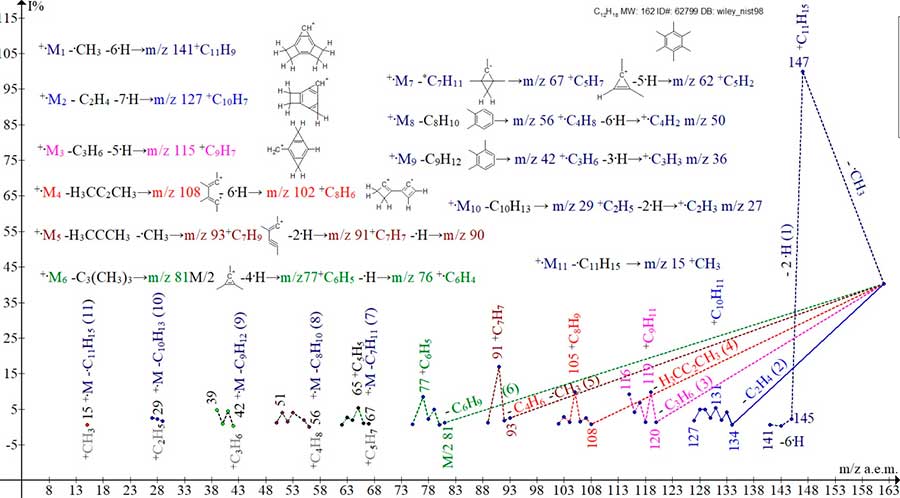
Figure 1. Eleven ion series of the mass spectrum of hexamethylbenzene C12 H18 MW: 162 ID#: 62799 DB: Wiley_nist98.
The first three series of the spectrum of hexamethylbenzene +.M1-3 (Fig.1.), including the detachment of groups: (.CH3 + 6 .H), (C2H4 + 7 .H) and (C3H6 + 5 .H) with the formation of +C11H9 ions, +С10H7 and +С9H7 are the result of fragmentation of methyl substituents occurring without rearrangement of the existing π-conjugations of the six-membered cycle. The four final series of +.M8-11 ions are formed upon detachment of neutral molecules C8H11 and C9H13, as well as radicals .C10H13 and .C11H15, in which the 6-membered ring is also retained. As a result of these four +.M8-11 series, +C4H7 m/z 55, +C3H5 m/z 41, +C2H5 m/z 29, and +CH3 m/z 15 ions arise.
Two intermediate series of ions +.M4-5 of hexamethylbenzene are formed upon detachments: dimethylacetylene CH3CCCH3 (-C4H6) conjugation π1-4, as well as dimethylacetylene with an additional, synchronous detachment of the methyl radical C4H6 + .CH3 (- .C5H9).
Two more series of +.M6-7 ions are the decomposition of +.M/2 in half (emission of the .C6H9 radical), the conjugation of π1-3, and the detachment of the rearrangement radical *.С7H11 (M/2 + CH2). In four fragment series +.M4-7, two pairs of ions are formed: +C8H12, *+C7H9 with conjugation π1-4 and+C6H9, *+C5H7 with conjugation π1-3.
Thus, in comparison with the benzene mass spectrum and five rearrangements of its π‑conjugations, only two rearrangements π1-4 and π1-3 are realized in the spectrum of hexamethylbenzene.
Ion series of the mass spectrum of hexakis(trifluoromethyl)benzene
In the spectra of n-perfluoroalkanes and perfluoropolycycloalkanes containing regular (CF2)n fragment groups, the variants of +.M decomposition pathways and the corresponding series of ions formed in parallel can be established from regular reference detachments. Usually, peaks of fluorocarbon ions with the same last significant figure of their mass are peaks of the same series of ions formed as a result of successive detachments of CF2. In the case when successive detachment of CF2 becomes impossible, either rearrangement detachment of CF2 occurs or elimination of another fragment group.
It should be noted that in the mass spectrum of trifluoromethylbenzene (NIST#: 118781 ID#: 134414 DB:
mainlib), the fragmentation of its trifluoromethyl group results in successive emissions of both
CF2 and .F, and .F and CF2,
which formally corresponds to the detachment of .CF3 radical.
The mass spectrum of hexakis(trifluoromethyl)-benzene (Fig.2.) consists of five ionic series M1-M5,
as well as two branches of the M1 and M3 series.
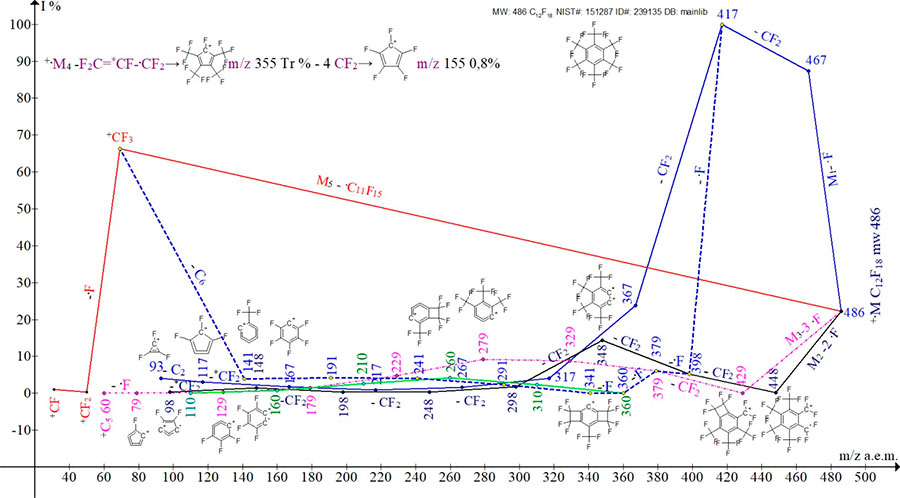
Figure 2. Five ion series of the mass spectrum of C12F18 hexakis(trifluoromethyl)benzene MW:486 NIST#: 151287 ID#: 239135 DB: mainlib.
The first three series of ions in the C12F18 spectrum are the result of three variants of primary detachment of fluorine atoms: detachment of one fluorine atom +.M1-F m/z 467, simultaneous detachment of two atoms +.M2-2F m/z 448 and simultaneous detachment of three fluorine atoms +.M3-3F m/z 429. As a result of subsequent regular emissions of CF2 (-50), three series of ions are formed, in which the last significant figures of their masses are: 7, 8 and 9. It should be noted that the simultaneous detachment of two fluorine atoms from two trifluoromethyl groups +.M2, as well as three fluorine atoms from three trifluoromethyl groups +.M3, seems unusual.
However, a similar primary detachment of three M-57 fluorine atoms, leading to the formation of a series of peaks of alkenyl ions +CF2(CF2)nCF=CF2, occurs during the fragmentation of n-perfluoroalkanes [4]. The detachment of fluorine from one of the CF3 groups (M-.F series) of perfluorotretbutylamine is also accompanied by the simultaneous detachment of two more fluorine atoms from the other two CF3 groups, so that the first peak of this series of the spectrum is the peak of the M-3.F ion m/z 614 4,1 % [5]. Simultaneous primary detachments of 3 fluorine atoms occur during the fragmentation of perfluorocyclohexane and perfluoropolycycloalkanes [6].
Series +.M1 of the mass spectrum of hexakis(trifluoromethyl)-benzene in Fig.2. marked with a solid blue line.
+.M1-.F +C12F17 m/z 467 (87,4%); -1CF2 +C11F15 417 (100%); -2CF2 367 (23,8%); -3CF2 317(4,1%); -4CF2 267 (1,6%); -5CF2 217 (0,9%); -6CF2 167 +C6F5 (1,8%); -*7CF2 117 +C5F3 (3,1%); -C2 93 +C3F3.
The final, seventh detachment of the rearrangement difluorocarbene *CF2 from +C 6F5 m/z 167, with the formation of +C5F3 m/z 117, is apparently the result of the energetically favorable formation of the five-membered cyclic ion +C5F3.
The ion of the series +.M1 +C11F15 m/z 417(100%) also fragments by successive detachments of four fluorine atoms and then by the emission of four CF2 to form an additional subset. The first series is branching. The last significant digit of the masses of the five ions in this subset is 1. The subset ends with the formation of the +CF3 ion. Subseries +.M1 in Fig.1. marked with a dotted blue line.
+.M1-.F -CF2 +C11F15 m/z 417 (100%); -1.F 398 +C11F14 (5,2%); - 2.F 379 +C11F13 (6,0%); -3.F 360 +C11F12 (0,1%); -4.F 341 +C11F11 (0,1%); -1CF2 291 +C10F9 (2,3%);
-2CF2 241 +C9F7(4,0%); -3CF2191 +C8F5(4,2%);-4CF2141 +C7F3(3,9%);-C6= +CF3;
Another branching of the M1 series occurs when the M1 series ion m/z 167 +C6F5 (1.8%) fragments both with the release of CF2 and with the removal of two fluorine atoms.
167 +C6F5 -.F =+C6F4 m/z148 ( 1,4%), -.F =+C6F3 m/z 129 (0.3%);
The second series of ions begins with the simultaneous emission of 2 fluorine atoms. It includes ions whose masses have the last significant figure 8. The +.M2 series in Fig.1. is marked with a solid black line.
+.M2-2.F +C12F16 m/z 448 (0.2%); -1CF2 +C11F14 398 (5,2%); -2CF2 +C10F12 348 (14,3 %); -3CF2 +C9F10 298 (5,2%); -4CF2 +C8F8 248 (1,7%); -5CF2 +C7F6 198 (0,3%); -6CF2 +C6F4 148 (1,4%); - C2 =124 +C4F4 (1,0%);
The detachment of the rearrangement *CF2 from the +C6F4 is completed by the +C5F2 ion.
m/z 148 (1,4%) +C6F4 -*7CF2 =+C5F2 m/z 98 (0,3%);
The third series of ions begins with the simultaneous emission of 3 fluorine atoms. Then, six consecutive CF2 detachments occur. The +.M3 series includes ions whose masses have the last significant figure of 9. The +.M3 series in Fig.1. marked with a solid purple line.
+.M3-3.F +C12F15 m/z 429(0.1%); -1CF2 +C11F13 379 (6,0%); -2CF2 +C10F11 329 (8,7%); -3CF2 +C9F9 279 (9,2%); -4CF2 +C8F7 229 (4,8%); -5CF2 +C7F5 179 (1,5%); -6CF2 +C6F3 129 (0,3%); - C3 = +C3F3 m/z 93 (4%);
The detachment of the rearrangement *CF2 from the +C6F3 is completed by the +C5F ion.
+C6F3 m/z 129 (0,3%); -*CF2 +C5F m/z 79 (0,1%);
The ion +C10F11 m/z 329 of the +.M3 series also fragments with the formation of an additional subset. It occurs as a result of two successive detachments of fluorine atoms, and then four successive emissions of difluorocarbene (M3 -3.F,-2CF2 -2.F -4CF2). The last significant digit of the ion masses of this subseries = 1. That is, the M3 series is branching. The subseries ends with the formation of the +C6F1 ion.
+C10F11 329(8,7%); -.F +C10F10 m/z 310 (2,3%); -.F +C10F9 m/z 291(2,3%); -1CF2 +C9F7 241(4,0%); -2CF2 +C8F5 191 (4,2%); -3CF2 +C7F3 141 (3,9%); -4CF2 +C6F1 91 (Tr);
Thus series 1 and series 3 have one common sub-series.
The fourth series of +.M4 ions appears as a result of the emission of the rearrangement perfluoroallyl radical *CF2=CF-.CF2 m/z 131. M4 series graph in Fig. 2. is not shown (only the fragmentation scheme is shown), since the peak intensities of this series are small and its plot overlaps with those of other ion series. The +.M4 series includes ions with masses whose last significant digit is 5. After five successive emissions of difluorocarbene, the series ends with the formation of the +C4F3 ion.
+.M4 -.C3F5 m/z131=355 +C9F13 (Tr);-1CF2 +C8F11 305(1%);-2CF2 +C7F9 255(4%);
-3CF2 +C6F7 205(8%); -4CF2 +C5F5 155(8%); -5CF2 +C4F3 m/z 105(5%);
The fifth variant of the +.M5 fragmentation is the detachment of the .C11F15 radical with the formation of the +CF3 ion m/z 69 (66.3%). The +.M5 series in Fig 1. is marked with a solid red line.
+.M5 C12F18 m/z 486 (22,2%) -.C11F15= +CF3 m/z 69 (66,3%);
Trifluoromethyl ion +CF3 m/z 69 (66.3%) arises both in the detachment of the -.C11F15 radical and in the decay of the fragment ion +C7F3 (3.9%) - C6 → +CF3 subseries +.M1.
Conclusion
Compared to the mass spectrum of benzene, which consists of 6 ionic series with five rearrangements of π-conjugations, the number of ionic series in the spectrum of hexamethylbenzene increases to eleven, but only two rearrangement variants are realized: π1-4 and π1-3. That is, methyl substituents make it difficult to rearrange π-conjugations, which is a consequence of a fifteenfold increase in their mass, compared with the mass of a hydrogen atom.
In contrast to the two ion series of the mass spectrum of hexafluorobenzene, the number of ion series in the spectrum of hexakis(trifluoromethyl)benzene increases to five. The series of hexafluorobenzene and hexakis(trifluoro)benzene ions do not confirm rearrangements of π‑conjugations. The reasons for this are the greater strength of π-bonds of fluorine-substituted carbon atoms of the cycle, compared with π-bonds of hydrogen-substituted benzene atoms, as well as the difference in the masses of substituents - methyl and trifluoromethyl groups.
An analysis of the ionic series of substituted benzenes makes it possible to establish fragmentation pathways, as well as the presence or absence of rearrangement of π-conjugations of the six-membered ring. The mass spectra of compounds presented as ionic series make it possible to determine and compare the total energies of competing fragmentation pathways.
Back in 1980, the opinion was expressed that the mechanism of ion formation remains practically unexplored [5]. The changes that have taken place since then have been the result of the emergence of new technical possibilities for recording mass spectra. This contributed to an increase in the number of surveys, the creation of libraries of mass spectra, as well as the loss of interest of researchers in mass spectra in terms of detailing the competing mechanisms of decay and formation of ion series. There was a belief that the era of organic mass spectrometry was over since most of the publications are now devoted to biomolecules.
Indeed, at present, mass spectrometers are more interested in the technical problems of obtaining mass spectra of complex biomolecules. That is, the focus of the study has shifted not so much to the mass spectrum itself and fragmentation processes, but to the fact of registration, as the main goal and main result of the study. Compared to the mass spectra of organic compounds, the mass spectra of biomolecules are more complex objects, which also reduce the interest in research of their fragmentation. However, the growing availability of NIST and WILE mass spectra libraries of both organic compounds and biomolecules allows the researcher to select the spectra of interest and try to understand the pathways of fragmentation processes.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of Russian Federation, using scientific equipment of INEOS RAS Molecule Structure Study Center.
References
- N. D. Kagramanov, Decay sequences - ion series of mass spectra of benzene, 1,3,5,7-cyclooctatetraene, [18]-annulene, hexafluorobenzene and its isomers, Fluorine notes, 2022, 3(142), 5-6.
- Ron, Arza; Hyams, I.J, Raman spectrum of crystalline hexamethylbenzene down to 2.deg.K., Chemical Physics Letters, 1972, 17(4), 557-60.
- Stride, John A., Determination of the low-temperature structure of hexamethylbenzene, Structural Science, 2005, B61(2), 200-206.
- Kagramanov N.D., Algorithms for the fragmentation of n-alkanes and n-perfluoroalkanes, Fluorine notes, 2020, 1(128), 3-4.
- Kagramanov N.D., Three series of mass spectrum ions of perfluorotributylamine (PFTBA), Fluorine notes, 2020, 3(130), 1-2.
- Kagramanov N.D., Series of fragment ions of cycloalkanes, perfluorocyclohexane, perfluoropolycycloalkanes, Fluorine notes, 2021, 3(136), 3-4.
- Khmelnitsky R.A., Lukashenko I.M., Brodsky E.S., Pyrolytic mass spectrometry of macromolecular compounds, M.: Chemistry, 1980, 280c, p.8.
ARTICLE INFO
Received 15 July 2022
Accepted 27 July 2022
Available online October 2022
Recommended for publication by PhD M. Manaenkova
eLIBRARY Document Number (EDN) JLDBTQ

Fluorine Notes, 2022, 144, 1-2
