Received: March 2023
DOI 10.17677/fn20714807.2023.03.01
Fluorine Notes, 2023, 148, 1-2
TRIFLUOROMETHYL-CONTAINING N-METHYLOLACRYLAMIDES FOR POLYMER PREPARATION
O. A. Melnik, A.A. Korlyukov, V.I. Dyachenko
A. N. Nesmeyanov Institute of Organoelement Compounds RAS, Russian Federation, 119334, GSP-1, Moscow, Vavilova st. 28, bld. 1
e-mail: omel@ineos.ac.ru
Abstract: By reacting acrylamide 1 with polyfluorocarbonyl compounds, stable CF3-containing N-hydroxymethylacrylamides with formula 3a-c were synthesized as monomers for the creation of polymeric materials for various purposes and their physicochemical properties were studied. Homo- and copolymers were obtained by free-radical (co)polymerization of 3a-c in solution, and their solubility, molecular weight characteristics and temperatures of glass transition were determined.
Keywords: acrylamide, polyfluoroketones, trifluoropyruvic acid esters, fluorine-containing N‑hydroxymethylacrylamides, free-radical (co)polymerization
Fluorine-containing polymers have valuable properties such as chemical, oxidation and thermal resistance as well as low refractive index, dissipation factor, dielectric constant and hydrophobicity. They are characterized by high resistance to atmospheric influences and long service life. Currently, they are widely used as coatings, fuel cell membranes, components for lithium-ion batteries, optical devices, components of composites and elastomers [1-5].
Fluorinated acrylic acid amides, as well as their homo- and copolymers, are characterized by a number of properties that their non-fluorinated counterparts do not possess. The prospects for using these compounds for self-assembly of various polymer architectures with unique properties are described in review [6]. The articles [7, 8] describe N-(2,2,2-trifluoroethyl)acrylamide, which has a strong red-shifted photoluminescence. It has been shown that its use as a comonomer with N,N‑dimethylaminoethyl methacrylate leads to formation of thermosensitive random copolymers capable of switching to both О2 and СО2 [9, 10].
Based on N-(2,2-difluoroethyl)acrylamide, the new biocompatible thermosensitive copolymers and nanogels containing a sufficient concentration of magnetically equivalent fluorine atoms for 19FMRT were synthesized [11].
Of the non-fluorinated acrylamide derivatives, N-hydroxymethylacrylamide (N-methylolacrylamide) (see Fig. 1) should be especially noted, which has been used in polymer chemistry for many decades to obtain polymers and copolymers with various compositions and purposes [12, 13].
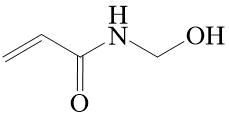
Figure 1. N-Methylolacrylamide.
Its copolymers have found application in electronics, pharmaceutical and textile industries, as well as in construction, and agriculture [14–16]. World production of this compound is currently hundreds of thousands of tons per year. Unfortunately, N-hydroxymethylacrylamide is unstable and at 20°С it starts to decompose into acrylamide and formaldehyde, and also transforms into N,N'‑methylenebisacrylamide) [17]. Therefore, for polymerization, as well as for transportation, it is usually used in the form of a 60% aqueous solution.
It should be noted that of all non-halogenated carbonyl compounds only formaldehyde forms with acrylamide a methylol derivative that is stable enough for further use (see Fig. 1). At the same time, it was stated that polyfluorocarbonyl compounds, such as polyfluoro(chloro)acetone and alkyl esters of trifluoropyruvic acid, react with amides of saturated, aromatic and heteroaromatic carboxylic acids, forming the stable alpha-polyfluoroalkyl-substituted methylol derivatives [18–20]. At present, only one example of reaction of acrylamide with hexafluoroacetone, leading to formation of compound with this structure, has been described in the literature [21].
The aim of this work is to study the interaction of acrylamide (1) with methyl (2a) and ethyl (2b) esters of trifluoropyruvic acid, leading to formation of alpha-trifluoromethyl-substituted N‑methylolacrylamides with formula (3), as well as to study their physicochemical properties and polymerization features.
It was found that the reaction of acrylamide 1 with methyl 2a or ethyl 2b esters of trifluoropyruvic acid occurs in anhydrous benzene upon short-term heating for 15 min of reagents with 10% excess of carbonyl compound (see Scheme 1).
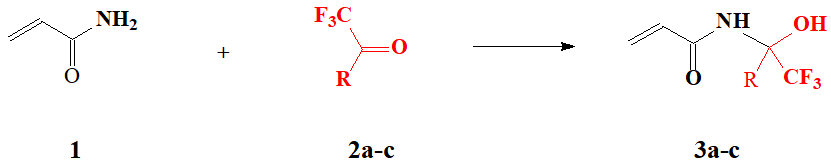
where R = CO2CH3 (2a, 3a), CO2C2H5 (2b, 3b), СF3(2c, 3c)
Scheme 1.
In this case, the yield of reaction products is 91–96%. Compounds 3a and 3b obtained are white crystalline substances, readily soluble in polar solvents such as dimethylformamide, ethyl acetate, acetone and tetrahydrofuran (THF). It should be noted that compounds 3a and 3b are registered in online database REGISTRY of STN information retrieval system as RN 355155-90-7 and RN 328270-27-5, respectively. However, neither the method of synthesis, nor the experimental NMR spectra, nor the physicochemical properties of these compounds are given in it.
To compare the reactivity of hexafluoroacetone 2с and trifluoropyruvic acid esters 2а, b in reaction with 1, as well as the tendency of obtained monomers as a result of these transformations to enter into polymerization, N-methylolacrylamide3с was synthesized. The interaction of 1 with 2c was also carried out in anhydrous benzene in a sealed glass ampoule at room temperature for 1 day. After opening the ampoule and removing the solvent, a spectrally pure white crystalline product 3c was obtained (with yield of 94%). In addition to highly polar solvents, the monomer 3c is also soluble on heating in toluene and dichloroethane.
The structure of compounds 3a-c was proved by results of elemental analysis, IR-, mass- and NMR-spectroscopy. In contrast to N-hydroxymethylacrylamide, compounds 3a-c do not change their physicochemical properties in air at room temperature throughout the year and, according to 1Н and 19F NMR spectra, remain specially pure.
Crystal structure of 3c was studied by X-ray diffraction study (Fig. 2). The geometry of acryamide fragment of 3с is planar, while the lengths of C1-N1, C1-O1, N1-C4, C4-C5, C4-O2 and C5-C6 (1.4558(13), 1.3800(11), 1.3557(12), 1.4732(15), 1.2460(12) and 1.3165(16) Å, respectively) indicate the absence of noticeable delocalization between double bonds of vinyl and carbonyl groups. The molecule of 3c contain strong intramolecular O-H...O bond (O1...O2 and O2...H1 distances are 2.6040(11) and 1.84 Å, respectively; O-H...O angle is equal to 149.5°).
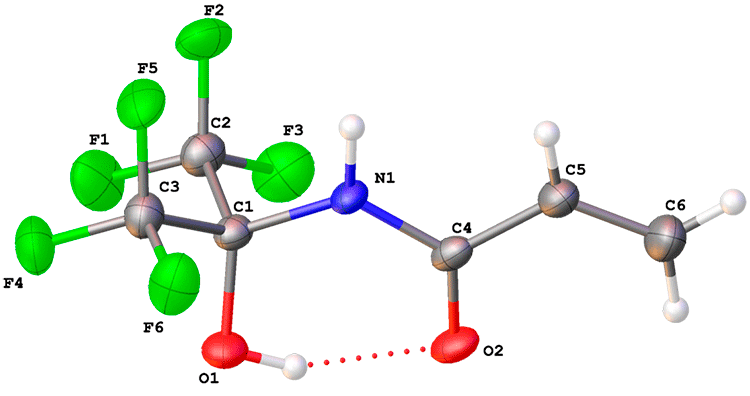
Figure 2. General view of 3c. Non-hydrogen atoms are presented as anisotropic displacement ellipsoids with probability are equal to 50%.
At the same time, the molecules of 3c are assembled into infinite chains via weak N-H...O bonds parallel to c axis of unit cell (Fig. 3).
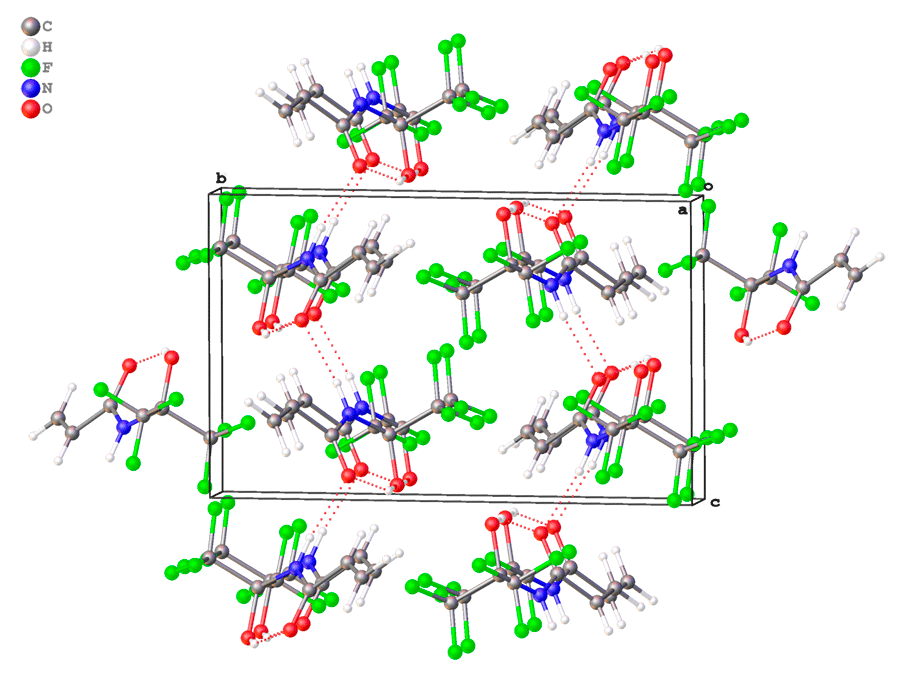
Figure 3. Crystal packing of 3c.
Free-radical polymerization of N-hydroxymethylacrylamides 3a-c and copolymerization of compound 3с with methyl methacrylate (MMA) were carried out in sealed glass ampoules in argon atmosphere in the presence of initiator -azobisisobutyric acid dinitrile (AIBN) in toluene or THF at 60°C for 4-8 h. The resulting (co)polymers were purified by reprecipitation with hexane from solutions in organic solvent.
The polymers are white powders, soluble in alcohols, dimethyl sulfoxide, N-methyl-2-pyrrolidone and insoluble in dimethylformamide, chloroform, benzene. Copolymers of compound 3c with MMA are soluble in chlorinated organic solvents, benzene, and toluene. The structure of (co)polymers was confirmed by elemental analysis, IR and NMR spectroscopy.
The IR spectra of these polymers contain the absorption bands corresponding to stretching vibrations of C-F (1230-1245,1110-1112, 1056 cm-1), N-H (3300-3200 cm-1), O-H (3500-3400 cm-1) bonds, C=O of amide (1680-1670 cm-1), C=O and C-O-C of ester group of polymers 3а and 3b (1760‑1750, 1261, 1200 cm-1). At the same time, there are no absorption bands in the spectra of stretching vibrations of C=C bond at 1645-1655 cm-1 and bending vibrations of C=C (975-965 cm‑1), which were present in IR spectra of initial monomers. IR spectra of these copolymers contain absorption bands characteristic for both MMA units and compound 3с: 1725 and 1144 cm-1 (С=О and С–О ММА), 1230 and 1110 cm-1 (СF3 of compound 3с).
1Н NMR spectra of polymers in D6-DMSO at 1.47-1.75 and 1.95-2.23 ppm contains two groups of broadened signals related to CH2 and CH fragments with integrated intensity of 2:1, respectively. PMR polymer spectra of compounds 3a and 3b also contain signals from ester groups with characteristic chemical shifts and intensity. The proton signals of CH2= fragments of compounds 3a–c are absent in spectra of these polymers. In the area of 7.52-9.51 ppm broadened signals related to protons of O-H and N-H groups are found. 19F NMR spectra contain broadened signals in the range -78.20 - -81.75 ppm.
The results of synthesis of (co)polymers in solution are presented in Table 1.
Table 1. Yield, molecular weight characteristics and temperatures of glass transition Tg for (co)polymers of compounds 3a-c ([AIBN] = 0.5 wt.%, [M] = 20 wt.%, reaction time 8 h, T = 60°C)
|
Monomer |
Solvent |
Yield of (co)polymer, % |
Mw, kDa |
Mw / Mn |
Tg, °C |
|
3а |
THF |
64 |
153 |
2,17 |
114 |
|
3b |
THF |
69 |
149 |
1,75 |
113 |
|
3с |
toluene |
67 |
145 |
1,54 |
112 |
|
3с + MMA* (20:80 mol. %) |
- |
72 |
320 |
1,20 |
100 |
*[AIBN] = 0.2 wt.%, reaction time 4 h
The yield of (co)polymers of compounds 3a-3c is 64-72%. The method of gel permeation chromatography (GPC) was used to evaluate their molecular weight characteristics. The molecular weights Mw of homopolymers are 145-153 kDa, and Mw for copolymer 3c with MMA is 320 kDa. The coefficient value of polydispersity of (co)polymers Mw / Mn lies in the range of 1.20-2.17, which is typical for radical polymerization. According results of thermomechanical analysis, the glass transition temperature Tg for polymers of compounds 3a-3c is practically independent of the nature of substituent R and is 112-114°C. The copolymer 3c and MMA has the same Tg as PMMA (100°C).
Previously, it was shown [22] that methylol derivatives analogous to 3а-с, obtained by reaction of hexafluoroacetone with acetamide or formamide, easily form stable crystalline complexes with alkaline earth metal ions and lanthanides.
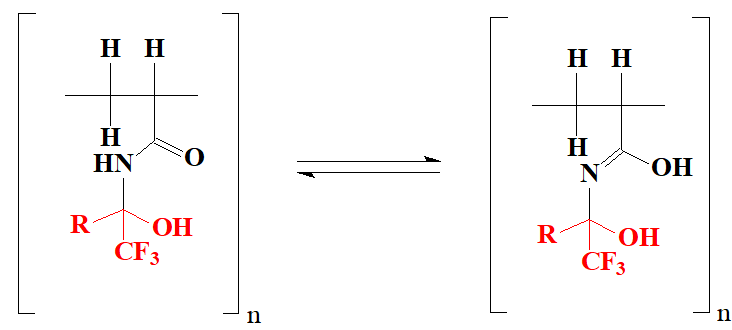
where R = CO2CH3(3a), CO2C2H5 (3b), CF3(3c).
Figure 4. Lactim-lactam tautomerism in a polymer matrix.
Apparently, the polymers obtained by us, being by their nature bidentate ligands (see Fig. 4), are also capable of possessing this property.
Thus, the synthesized (co)polymers based on monomers 3a–3c can serve as a matrix for creating a number of composite, ion- and electrically conductive materials.
Experimental part
The experiments used industrially available starting reagents - acrylamide [CAS 79-06-1], hexafluoroacetone 98% [CAS 684-16-2], trifluoropyruvic acid methyl ester 98% [CAS 13089-11-7], trifluoropyruvic acid ethyl ester 98 % [CAS 13089-18-0]. MMA (Aldrich, 99%) was distilled under reduced pressure.
1H and 19F NMR spectra were recorded in D6-DMSO (copolymers with MMA - in CDCI3) via Bruker Avance 300 instrument (at frequencies of 300 and 282 MHz, respectively). Chemical shifts in NMR spectra are given in δ (ppm) scale relative to TMS as internal standard (for 1H NMR spectra) and CCl3F as external standard (for 19F NMR spectra). The spin-spin interaction constants are given in Hz. IR spectra were recorded via Bruker Vertex 70 v Fourier spectrometer (with resolution 4 cm–1 in frustrated total reflection mode) using a PIKE Glady ATR attachment with diamond working element. LC-MS analysis of obtained compounds was carried out via Shimadzu LCMS-2020 high-performance liquid chromatograph-mass spectrometer using DUIS ionization method and acetonitrile (HPLC gradient 99.9+%, Chem-Lab) as mobile phase and solvent.
X-ray crystallographic study
Crystals of 3c are monoclinic (space group P2₁/n), chemical composition C₆H₅F₆NO₂, a = 8.5937(7), b = 12.9117(11), c = 9.1073(8) Å, β = 115.812(3), V = 909.72(14) ų, Z = 4, dcalc= 1.731 g·cm-3, F(000) = 472, M = 237.11. Single crystal (colourless prism-shaped, dimensions 0.21 × 0.24 × 0.38 mm) was selected and intensities of 14254 reflections were measured with Bruker QUEST diffractometer at 100K (ϕ and ω scans, microfocus sealed X-ray tube, λ[MoKα] = 0.71073 Å, μ = 0.205 mm-1, 2θmax = 66.442°). After merging of equivalents and absorption correction 3452 independent reflections (Rint = 0.0343) were used for the structure solution and refinement. The structure was solved by direct method and refined by full-matrix technique against F2 in anisotropic approximation. The positions of hydrogen atoms in methyl and methylene groups were calculated geometrically and refined in rigid body approximation. Final R factors: R1 = 0.0382, (2068 reflections with I>σ(I)), wR2 = 0.0909 (all reflections), GOF = 0.981. The structure was solved with the ShelXT program [23] and refined with the ShelXL program [24]. Molecular graphics was drawn using OLEX2 program [25].
CCDC 2266082 contains the supplementary crystallographic data for 3c. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures
The molecular weights of (co)polymers were determined by GPC method in N-methyl-2-pyrrolidone (at flow rate of 1 ml/min and temperature of 25°C) via Knauer Smartline instrument equipped with Phenomenex Phenogel10E4A column (300 x 7.8 mm, 5 µm). This column was calibrated using polystyrene standards and refractometer as detector. The temperatures of glass transition were determined using thermomechanical analysis via TMA Q400 analyser (company TA Instruments) equipped with probe 2.54 mm in diameter and at load of 100 g and sample heating rate of 5°C/min (in temperature range of 20-250°C).
Methyl ester of 2-acryloylamino-2-hydroxy-3,3,3-trifluoropropionic acid (3a)
The acrylamide 0.71 mg (10 mmol) and 12 ml of anhydrous benzene were placed in a 50 ml glass three-necked conical flask equipped with heated magnetic stirrer, thermometer, addition funnel and reflux condenser. Turned on heating and stirring. When the acrylamide solution reached a temperature of 80°C, 1.74 g (11 mmol) of trifluoropyruvic acid methyl ester was added to it in one step and the reaction mixture was boiled for 10 min. Then the heating and stirring was turned off. After spontaneous decrease in temperature a plentiful precipitate fell out and complete crystallization of the product the reaction mass was left overnight at 20°C. The precipitate formed was filtered off, washed with benzene and dried at the glass filter with air to constant weight. 2.2 g of white crystalline substance 3a was obtained. Yield 97%, m.p. 125-126°C (benzene).
Founded, %: С 36.94; H 3.57; F 25.02. С7Н8F3NO4. Calculated, %: С 37.02; H 3.55; F 25.09.
Mass spectrum, m/z: 227 [M+].
1H NMR spectrum (300 MHz, D6-DMSO, δ, ppm, J/Hz): 9.53 (s, 1H, NH), 7.99 (s, 1H, OH), 6.39 (d.d., 1H, CH, 3JH-H(cis)=12, 3JH-H(trans)=9), 6.17 (dd, 1H, H2C=, 3JH-H=12, 2JH-H=3), 5, 73 (dd, 1H, H2C=, 3JH-H=9, 2JH-H=3), 3.70 (s, 3H, OCH3).
19F NMR spectrum (282 MHz, D6-DMSO, δ, ppm): -78.83 (s, 3F, CF3).
Ethyl ester of 2-acryloylamino-2-hydroxy-3,3,3-trifluoropropionic acid (3b)
The acrylamide 0.35 g (5 mmol) and 6 ml of anhydrous benzene were placed in a glass pear-shaped flask equipped with heated magnetic stirrer, thermometer, addition funnel and reflux condenser. Turned on heating and stirring. Upon reaching 80°C, 1.0 g (5.9 mmol) of ethyl ester of trifluoropyruvic acid was added to this flask in one step. The reaction mixture was boiled for 15 min, then heating and stirring were turned off and this mixture was allowed to spontaneously cool to 20°C. At the same time, the formation of white precipitate was observed. The reaction mass was left overnight at room temperature; the next day the precipitate was filtered off, washed with benzene and dried at glass filter to constant weight. 1.1 g of white crystalline substance 3b was obtained. Yield 91%, m.p.96-97°C (benzene).
Founded, %: С 39.94; H 4.29; F 23.43; N 5.82. С8Н10F3NO4. Calculated, %: С 39.84; Η 4.18; F23.63; N 5.81.
Mass spectrum, m/z: 241 [M+].
1H NMR spectrum (300 MHz, D6-DMSO, δ, ppm, J/Hz): 9.50 (s, 1H, NH), 7.88 (s, 1H, OH), 6.39 (dd, 1H, CH, 3JH-H(cis)=15, 3JH-H(trans)=9),6.16 (dd, 1H, H2C=, 3JH-H=15, 2JH-H=3), 5,72 (dd, 1H, H2C=, 3JH-H= 9, 3JH-H= 3), 4.17 (m, 2H, OCH2, 3JH-H=60), 1.17 (t, 3H, CH3, 3JH-H= 60).
19F NMR spectrum (282 MHz, D6-DMSO, δ, ppm): -78.73 (s, 3F, CF3).
N-(2,2,2-trifluoro-1-hydroxy-1-trifluoromethylethyl)acrylamide (3c)
The acrylamide 2.13 g (30 mmol) of acrylamide and 30 ml of anhydrous benzene prepared by azeotropic distillation of water and subsequent distillation were placed in a 50 ml glass ampoule. This ampoule was cooled with a mixture of acetone and dry ice to -78°C, and 7.5 g (45 mmol) of hexafluoroacetone (b.p. -28°C) was condensed into it. Then it was soldered, allowed to spontaneously heat up to 20°C and kept for 1 day under these conditions. The ampoule was again cooled to -78°C, opened, heated to room temperature, while the excess of hexafluoroacetone was condensed into a trap. The reaction mass was transferred from this ampoule to a conical flask and evaporated at rotary evaporator. The residue in the form of white crystalline substance was removed from the flask to filter funnel and washed with cold petroleum ether. 6.7 g of white crystalline, spectrally pure compound 3c were obtained. Yield 94%, m.p. 48-49°C
Founded, %: С 30.17; H 2.28; F 47.96. С6Н5F6NO2. Calculated, %: С 30.39; H 2.13; F 48.08.
Mass spectrum, m/z: 237 [M+].
1H NMR spectrum (300 MHz, D6-DMSO, δ, ppm, J/Hz): 9.58 (br.s, 1H, NH), 9.47 (s, 1H, OH), 6.51 (dd, 1H, CH,2JH-H=18, 3JH-H=12), 6.27 (dd, 1H, H2C=,2JH-H=18,2JH-H=3), 5.82 (dd, 1H, H2C=, 2JH-H=12, 2JH-H=3).
19F NMR spectrum (282 MHz, D6-DMSO, δ, ppm): - 78.20 (s, 6F, 2CF3).
Preparation of 2-acryloylamino-2-hydroxy-3,3,3-trifluoropropionic acid polymethyl ester
To a solution of 2.30 g of compound 3a in 11 ml of THF was added 0.012 g (0.5 wt.%) AIBN as a polymerization initiator. The prepared reaction mass was filtered into a glass ampoule, which was then filled with argon, sealed and placed in a thermostat. Polymerization temperature is 60°C. After 8 h this ampoule was removed, cooled and opened. The resulting polymer was purified by reprecipitation from a solution in THF into hexane, washed repeatedly with hexane and dried in vacuum at 40°C for 72 h. Polymer yield (white powder) 1.47 g (64%).
Founded, %: С 37.24; H 3.38; F24.89; N 6.22. Calculated, %: С 37.02; H 3.55; F25.09; N 6.17.
19F NMR spectrum (D6-DMSO, δ, ppm): -78.28 – -79.78 (br.s, 3F, CF3).
Preparation of polyethyl ester of 2-acryloylamino-2-hydroxy-3,3,3-trifluoropropionic acid
Synthesized similarly to above method from 2.55 g of compound 3b, 12 ml of THF and 0.013 g (0.5 wt.%) AIBN. Polymer yield 1.76 g (69%).
Founded, %: С 40.05; Η 4.03; F23.31; N 5.96. Calculated, %: С 39.84; Η 4.18; F23.63; N 5.81.
19F NMR spectrum (D6-DMSO, δ, ppm): -78.76 – -80.09 (br.s, 3F, CF3).
Preparation of poly[(N-(2,2,2-trifluoro-1-hydroxy-1-trifluoromethylethyl)acrylamide]
Synthesized analogously to above method from 2.79 g of compound 3c, 13 ml of toluene and 0.014 g (0.5 wt.%) AIBN. Polymer yield 1.87 g (67%).
Founded, %: С 30.68; Η 2.06; F 47.73; N 6.08. Calculated, %: С 30.39; H 2.13; F48.08; N 5.90.
19F NMR spectrum (D6-DMSO, δ, ppm): -78.20 – -80.25 (br.s, 6F, 2CF3).
Preparation of copolymer of methyl methacrylate and N-(2,2,2-trifluoro-1-hydroxy-1-trifluoromethylethyl)acrylamide at initial monomer ratio of 80:20 mol.%
Similarly, from 1.32 g of MMA, 0.92 g of compound 3c and 0.0045 g (0.2 wt.%) AIBN after heating at 60°C for 4 h, a transparent solid colorless copolymer was obtained. It was purified by reprecipitation from solution in THF into hexane, washed several times with hexane and dried in vacuum at 40°C for 72 h. Yield 1.61 g (72%). The ratio of units in this copolymer is 83:17 mol.% (determined by content of fluorine).
Founded, %: С 47.87; H 6.03; F14.60; N 1.88. Calculated, %: С 45.95; H 5.45; F 16.79; N 2.06.
19F NMR spectrum (CDCI3, δ, ppm): -81.00 – -81.75 (br.s, 6F, 2CF3).
Conclusions
Fluorine-containing N-hydroxymethylacrylamides have been synthesized, which can be effectively used as monomers to obtain polymer composite and ionogenic materials of various architectures with gas separation, electrical conductivity and other useful functional properties. The presence of N-hydroxymethylamide group in addition to double bond of compounds 3a–3c also makes it possible to consider their use as crosslinkers. The (co)polymers of these compounds were obtained by free-radical (co)polymerization. Their molecular weight and thermal characteristics were determined.
Acknowledgments
This work was carried out within the framework of State Assignment No. 075-03-2023-642 from Ministry of Science and Higher Education of Russian Federation using the scientific equipment of Molecules Structure Study Center of INEOS RAS. The authors thank M. M. Ilyin for determination of molecular weight characteristics of polymers, and E. S. Afanasyev - for performing thermomechanical analysis.
References
- Fluoropolymers, ed. Knunyants I. L., Ponomarenko V. A., M.: Mir Publishing house, 1975, 448 p. (in Russian)
- Hougham G., Cassidy P.E., Jones K., Davidson T., Fluoropolymers: Synthesis and applications. New York: Plenum Publishers, 1999, v. 1-2.
- Fluoropolymer Materials, Ed. Buznik V. M., Tomsk, NTL Publishing house, 2017, 600 p. (in Russian)
- Améduri B., Boutevin B., Well architecture fluoropolymers: Synthesis, properties and applications. Amsterdam, Elsevier, 2004, р. 231–348.
- Améduri B., The promising future of fluoropolymers. Macromol. Chem. Phys, 2020, 221(8), 1900573.
- Guerre M., Lopez G., Améduri В., Semsarilar М., Ladmiral V., Polym. Chemistry, 2021, 12 (27), 3852-3877.
- Halpern А.et al., U. S. Atomic Energy Comm., WADC-TR 54-264, 1954, 74.
- Long J. et al., Chemistry An Asian Journal, 2021, 16(17), 2426-2430.
- Lei L., Qi Zhang, Shuxian S., Shiping Z., Polym. Chemistry, 2016, 7(34), 5456-5462.
- Steglich W. et al., Chemische Berichte, 1974, 107, 1488-1498.
- Kolouchova K., Sedlacek O., Jirak D. et al., Biomacromolecules, 2018, 19(8), 3515-3524.
- Feuer H., Lynch U. E., J. Am. Chem. Soc., 1953, 75(20), 5027-5029.
- Patent CN112939799А, 2021.
- Мa L., Zhang Y., Wang X. et al., J. Applied Electrochemistry, 2021, 51(2), 131-141.
- Arslan M.et al., Polym. Bulletin, 2021, 78(3), 1535-1550.
- Baysak F. K., Isiklan N., J. Applied Polym. Sci., 2022, 139(16), art. no. 51976.
- Gur’eva L. L., Tkachuk A. I., Estrin Ya. I. et al., Polymer Science, Series А, 2008, 50(3), 283‑290. (in Russian)
- US Patent 3324178, 1967.
- Osipov S. N., Kolomiets A. F., Fokin A. V., Bulletin of USSR Academy of Sciences, Div. of Сhemical Sciences, 1988, 3, 122-126.
- Aksinenko A. Yu., Pushin A. N., Sokolov V. А., Russian Chemical Bulletin, 2002, 51(11), 2136‑2138.
- US Patent US3549705, 1970.
- MacDonald С., Willis C. J., Can. J. Chem., 1973, 51, 732-740.
- Sheldrick G.M., Acta Cryst.A71,2015, 3-8.
- Sheldrick G.M., Acta Cryst.C71, 2015, 3-8.
- Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H., J. Appl. Cryst., 2009, 42, 339-341.
ARTICLE INFO
Received 20 March 2023
Accepted 25 April 2023
Available online June 2023
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) EIUHJY

Fluorine Notes, 2023, 148, 1-2
