Received: October 2023
DOI 10.17677/fn20714807.2023.05.01
Fluorine Notes, 2023, 150, 1-2
SYNTHESIS OF PERFLUOROHEX-1-ENE HOMOPOLYMER AT ULTRAHIGH PRESSURE
V.I. Sokolov1,2, S.A. Akhmanov1,2, I.O. Goryachuk1, E.V. Polunin3
1Federal Scientific Research Center "Crystallography and Photonics" RAS, 119333, Leninsky ave. 59, Moscow, Russia
2 Scientific Research Institute of System Analysis RAS, Nakhimovsky ave. 36, 1, 117218 Moscow, Russia
3N. D. Zelinsky Institute of Organic Chemistry RAS, 119991, Leninsky ave. 47, Moscow, Russia
Abstract: Perfluorohex-1-ene homopolymer CF2 = CF – (CF2)3 – CF3 was synthesized using the ultrahigh pressure method (15-16 thous. atm). The resulting homopolymer is partially crystalline, has normal dispersion and a refractive index n = 1.334-1.340 in visible wavelength range λ= 440-672 nm. It is soluble in perfluorinated solvents, is capable of film formation and can be used to create various polymer devices.
Keywords: perfluorinated polymers, perfluorohex-1-ene, ultrahigh pressure polymerization, refractive index.
Introduction
Among perfluorinated compounds containing double C=C bonds and potentially capable of radical polymerization, there are a number of monomers that, under normal conditions, polymerize with great difficulty due to steric hindrance. These include perfluorostyrene [1], perfluoroisopropyl vinyl ether [2], as well as olefins of homologous series CF2 = CF - Rf, where Rf = (CF2)m – CF3 is an aliphatic perfluorinated radical, m = 1, 2, ... . So, the hexafluoropropylene homopolymer CF2 = CF – CF3 was first obtained by radical thermal polymerization using ultrahigh pressure P = 6 -12 thous. atm. at temperature T = 230 – 290 °C [3]. This article reports on synthesis at ultrahigh pressure (15-16 thous. atm.) of homopolymer from another monomer of this series: perfluorohex-1-ene CF2 = CF – (CF2)3 – CF3. Data on structure and refractive index of this polymer in visible spectral region are presented.
Synthesis of polyperfluorohex-1-ene at ultrahigh pressure
To obtain the perfluorohex-1-ene homopolymer, the corresponding monomer CF2=CF-(CF2)3–CF3 (H1) produced by company P&M Invest (Russia) was used. Monomer H1 is a transparent, colorless liquid with boiling point b.p. = 56-58°C and refractive index nD = 1.272. The purity of this monomer exceeded 88%, and the main impurity, according to manufacturer, was perfluoro(4-methylpent-2-ene) CF3-CF=CF-C(CF3)2F. Synthesis of homopolymer H1 was carried out in Teflon ampoules with diameter of 14 mm and volume of 1–2 ml in cylinder-piston molds at a pressure of 15-16 thous. atm. and temperature 180-240°C. Reaction times ranged from 168 to 336 h. Before synthesis, the monomers were distilled in an argon atmosphere to remove dissolved oxygen, which is known to be an inhibitor of radical polymerization reaction.
Preliminary experiments on the polymerization of perfluorohex-1-ene at ultrahigh pressure, carried out without using of any initiators, did not lead to formation of a polymer. Therefore, further synthesis was carried out using the perfluorinated peroxide initiator based on pentafluorobenzoic acid, the molar concentration of which was 1.5-2.2%. The synthesis scheme is shown in Figure 1. The product obtained after the end of this reaction was a viscous transparent or slightly colored gel containing, in addition to the linear homopolymer, highly volatile components (for example, unreacted monomers of perfluorohex-1-ene and perfluorohex-2-ene), as well as various reaction by-products (dimers, oligomers, etc.). In order to remove these substances, the homopolymer was evacuated to constant weight at a pressure of 15 mbar and a temperature of 100°C. The yield of homopolymer in these experiments was about 30%.
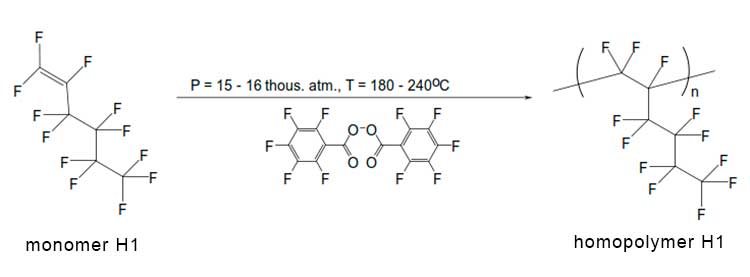
Figure 1. Scheme for synthesis of perfluorohex-1-ene homopolymer H1 by radical polymerization at ultrahigh pressure using a perfluorinated peroxide initiator. n is the number of units in the homopolymer macromolecule.
Figure 2 shows 19F NMR spectra of monomer H1 and its homopolymer dissolved in hexafluorobenzene, obtained via Bruker AM-300 spectrometer at 282.40 MHz.
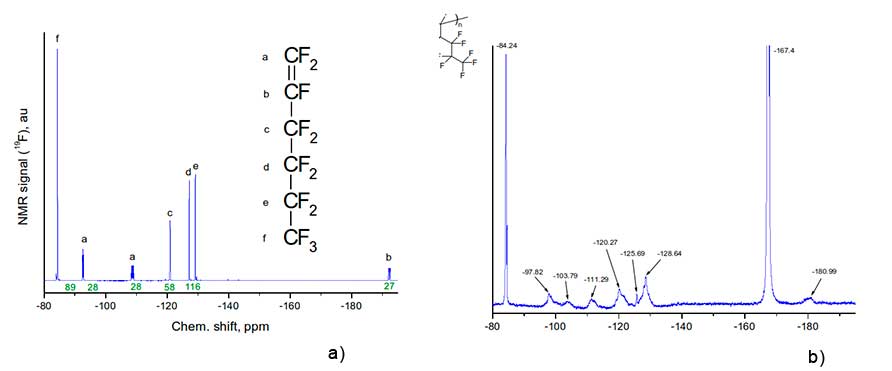
Figure 2. 19F NMR spectra of monomer H1 (a) and corresponding homopolymer (b), obtained via Bruker AM-300 spectrometer at 282.40 MHz) The insets show the structure of monomer and the fragment of structure of its homopolymer.
The assignment of signals in 19F NMR spectrum of monomer H1 to corresponding groups of atoms a, b, c, d, e, f is given in Figure 2a. Let us analyze the positions of 19F NMR signals and their assignment to molecular groups for homopolymer H1 (see Figure 2b). First of all, we note that the signal is -167.4 ppm in this figure is associated with solvent - hexafluorobenzene in which the homopolymer was dissolved. Broadened singlet at -84.24 ppm corresponds to three fluorine atoms in trifluoromethyl group in the side substituent of polymer macromolecule (this signal is also visible in Figure 2a). At the same time, the signal nearby is 192.2 ppm, due to the presence of a double C=C bond in monomer molecules (see signal “b” in Figure 2a), in Figure 2b is missing. This indicates the absence of such bonds in synthesized homopolymer.
To estimate the molecular weight of synthesized homopolymer, the average hydrodynamic diameter of its macromolecular globules in perfluorooctane (nD = 1.255) was measured. Measurements were performed by dynamic light scattering method using a 90Plus_Zeta particle/protein analyzer (Brookhaven Instruments Corp., USA) illuminated by a 640 nm laser beam.
Figure 3 shows the histogram of globules size distribution. It can be seen that average diameter of these globules is <D> = 5.4 nm. Thus, the homopolymer H1, synthesized by radical polymerization at ultrahigh pressure, can be classified as a medium-high molecular weight substance.

Figure 3. Histogram of size distribution of homopolymer H1 macromolecular globules, measured by dynamic light scattering method in perfluorooctane, where D is diameter of these globules. The insets show the form of autocorrelation function C(τ), where τ- is time in ms, as well as a fragment of homopolymer structure.
Study of homopolymer structure using wide-angle X-ray scattering method
Synthesis of perfluorinated homo- and copolymers at ultrahigh pressure occurs by a radical mechanism [4, 5], and both amorphous and partially crystalline materials can be formed. Structural diagnostics of homopolymer H1 was carried out via wide-angle X-ray diffractometer Rigaku Miniflex600 (Cu,λ= 1.54184 A) in 2θ angle range from 3 to 90 deg. (with a step of 0.02 deg.). The diffraction pattern of the film deposited to a glass substrate by drying a solution of homopolymer H1 in perfluorodecalin is shown in Figure 4. As follows from Figure 4, wide “halos” in diffraction pattern near 2θ ≈ 7.5, 16.4, 40.4 and 76.4 deg. are observed. In addition to these halos, sharp peaks are also visible at 2θ ≈ 10.68, 20.73, 31.71 and 37.16 deg. This type of X-ray diffraction pattern indicates the partial crystallinity of studed material. Note that freshly deposited H1 homopolymer films are transparent. However, over time they acquire some dullness, which may be a consequence of formation of polycrystallites (lamellas).
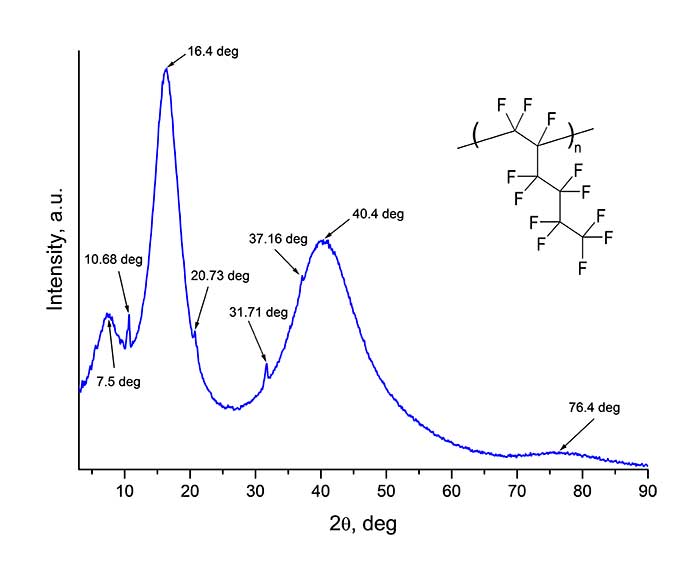
Figure 4. X-ray diffraction pattern of a film of homopolymer H1 obtained via Rigaku Miniflex600 X-ray diffractometer. θ is the angle of incidence of X-ray beam to the sample.
It should be noted that the synthesized perfluorinated homopolymer H1, although partially crystalline, nevertheless dissolves at room temperature in perfluorinated solvents, such as perfluorohexane, perfluorooctane, hexafluorobenzene, perfluorocarbogal, perfluorodecalin. Its copolymers with other perfluorinated monomers (for example, with perfluoro-2,2-dimethyl-1,3-dioxole), as our studies have shown, are amorphous. Such perfluorohex-1-ene copolymers are transparent in visible and near-IR wavelength ranges and can be used to create various integrated optical devices, for example, optical waveguides, high-speed waveguide modulators and optical radiation amplifiers.
Measurement of the refractive index of homopolymer H1 by spectroscopic refractometry
Study of refractive index n and dispersion dependence n(λ) of homopolymer H1 was carried out by method of spectroscopic refractometry [6] in wavelength range λ= 440-672 nm using a specialized complex created on the basis of Atago M1550/D2 multi-wavelength refractometer (Atago, Japan). To do this, a solution of homopolymer H1 in perfluorocarbogal was applied in a form of layer ≥1 mm thick onto measuring prism of refractometer and kept at room temperature until the solvent completely evaporated. After this, the upper surface of polymer layer was matted to ensure uniform illumination of field of view of this refractometer. The monochromatic sample illumination system consisted of a broadband light source (250 W halogen lamp), an M266 monochromator (SOLAR Laser Systems, Belarus) and a multicore fiber optic cable. The measurement results are presented in Figure 5.

Figure 5. Dispersion dependence of refractive index n(λ) of homopolymer H1, measured by spectroscopic refractometry method. Crosses are the experimental data and solid line – the approximation by second degree polynomial of n(λ) = A + Bλ + Cλ2, where A = 1.3788, B = -1.287 x 10-4 nm-1, C = 9.155 x 10-8 nm-2.
As follows from Figure 5, the homopolymer H1 has a normal course of dispersion, and its refractive index in wavelength range λ= 440-672 nm varies from n = 1.340 to 1.334.
Conclusion
A homopolymer was synthesized using the ultrahigh pressure method from perfluorohex-1-ene monomer in the presence of a perfluorinated peroxide initiator. It dissolves in perfluorinated solvents and has a refractive index n = 1.334-1.340 (in visible wavelength range λ= 440-672 nm). The resulting homopolymer is partially crystalline, but its copolymers with other perfluorinated monomers (e. g. perfluoro-2,2-dimethyl-1,3-dioxole) are amorphous. Such copolymers can be used to create various integrated optical devices, for example, waveguide modulators and compact waveguide amplifiers for telecommunications C-band wavelengths 1530-1565 nm.
Acknowledgments
This study was financially supported by Ministry of Science and Higher Education RF within the framework of state assignment to Federal Research Center “Crystallography and Photonics” RAS. In this study was used the equipment of this Federal Research Center (Center for Collective Use).
References
- L. A. Wall, D. W. Brown. High pressure polymerization of perfluorostyrene. Journal of Fluorine Chemistry, 1972, 2, 73-85.
- E. V. Polunin, S. I. Molchanova, J. E. Pogodina, V. I. Sokolov, I. V. Zavarzin, Homo- and co-polymerisation of perfluoroisopropylvinyl ether under high pressure, Fluorine Notes, 2017, 114(5), 5-6.
- A. A. Zharov, I. A. Guzyaeva, Kinetics and mechanism of thermal polymerization of hexafluoropropylene under high pressures, Russian Chemical Bulletin, 2010, 59(6), 1225-1231.
- V. I. Sokolov, V. E. Boyko, I. O. Goryachuk, S. M. Igumnov, S. I. Molchanova, Yu. E. Pogodina, E. V. Polunin, Synthesis and optical properties of copolymers of perfluoro-2,2-dimethyl-1,3-dioxole and perfluoropropyl vinyl ether, Russian Chemical Bulletin, International Edition, 2017, 66(7), 1284-1289.
- V. I. Sokolov, I. O. Goriachuk, I. V. Zavarzin, S. I. Molchanova, Yu. E. Pogodina, E. V. Polunin, and A. A. Yarosch, New copolymers of perfluoro-2-ethyl-2-methyl-1,3-dioxole and perfluorovinyl ether with low non-monotonic refractive index, Russian Chemical Bulletin, International Edition, 2019, 68(3), 559-564.
- Sokolov V. I., Savelyev A. G., Bouznik V. M., Igumnov S. M., Khaydukov E. V., Molchanova S. I., Tuytuynov A. A., Akhmanov A. S., Panchenko V. Ya., Refractive index and dispersion of highly-fluorinated acrylic monomers in the 1.5 m telecom wavelength region measured with a spectroscopic Abbe refractometer, Measurement Science and Technology, 2014, 25(7), 077001.
ARTICLE INFO
Received 03 October 2023
Accepted 17 October 2023
Available online October 2023
Recommended for publication by PhD V. Don
eLIBRARY Document Number (EDN) RMGIWH

Fluorine Notes, 2023, 150, 1-2
