Received: October 2023
DOI 10.17677/fn20714807.2023.05.03
Fluorine Notes, 2023, 150, 5-6
THE REACTION OF POLYFLUOROKETONES WITH DIMECARBIDE – A NEW APPROACH TO 6-FLUOROALKYLMODIFICATION OF 5-HYDROXYINDOLES
V.I. Dyachenko, S.M. Igumnov
A.N. Nesmeyanov Institute of Organoelement Compounds RAS, Russian Federation, 119334, Moscow, Vavilov str. 28/1.
e-mail: vic-d.60@mail.ru
Abstract: Hexafluoroacetone 2а, nitropentafluoroacetone 2b and perfluorocyclohexanone 2с are smoothly reacting with dimecarbide 1 to form products of С-6-oxyalkylation (5a-c) with yields of 74-90%. In the case of trifluoro- 3 and difluorochloropyruvic acid methyl esters 4, the oxyalkylation reaction is accompanied by cyclization of the intermediate esters 6а, 7а into the corresponding lactones 6b,7b.
Keywords: serotonin, 5-oxyindoles, hexafluoroacetone, nitropentafluoroacetone, methyltrifluoropyruvate, methyldifluorochlorpyruvate, perfluorocyclohexanone, dimecarbine, C-oxyalkylation
5-Oxyindole derivatives (5-OI) occupy a special place in the physiology of the higher nervous system. Serotonin plays an important role of the neurotransmitter of the human CNS by regulating the transmitting pulses processes in the neurons of the cardiovascular, endocrine and other physiologists of important body systems [1, 2]. The similar to it by the structure, hormone melatonin is involved in the change control in the day and night rhythms of living organisms [3]. The synthetic derivatives of 5-OI – indomethacin and dimecarbine, for many years are used in medical practice as an anti-inflammatory and hypotensive agent, respectively [4]. The domestic Arbidol medication strongly holds the position on the market of antiviral medications [5,6].
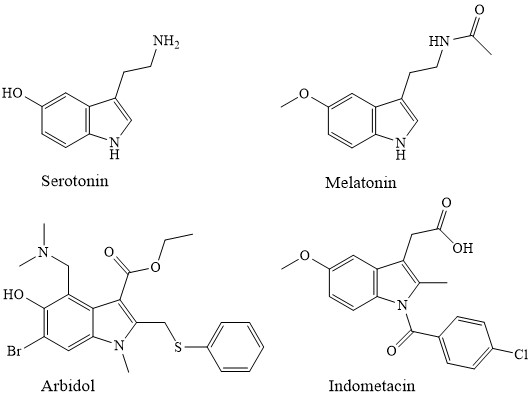
5-OI, being indoles on one side, and with the other side - phenols, have an enriched chemistry. The great contribution to its development is introduced by Grinev et al. The most typical are electrophilic substitution reactions: alkylation, acylation, halogenation, diazotization, and various aminomethylation variants [7].
It has been established that the presence of fluorine in biologically active compounds significantly affects the metabolism [8] thereof. The presence of fluorine or polyfluoroalkyl groups in the molecule increases the lipophilic property of the fluorine-containing compounds, thus making it easier to penetrate the cell protein-lipid membrane. The comparison by volume of a fluorine atom with a hydrogen atom in organic compounds, as well as by nucleophilicity with an oxygen atom, formed the basis called “the masking effect” [9]. Consequently, at present there is an increasing interest in the development of novel methods for the synthesis of fluorine-containing compounds, and more particularly to the treatment of CF3-containing indoles, which structure is based on the compounds studied in the present work [10]. Despite the considerable success in synthetic organic chemistry, no methods of single-step introduction of fluorine-containing substituents in 5-OI in the literature are described.
Having studied the patterns of reactions of polyfluorocarbonyl compounds with phenols, naphthols, polyphenols, phenolates, 8-oxyquinolines [11], we found the convenient method of one-step fluoroalkyl modification of 5-ОI on the example of reaction of polyfluoroketones with ethyl ester of 5-oxy-1,2-dimethyl-1H-indole-3-yl carboxylic acid 1 - dimecarbine [4]. In these transformations, hexafluoroacetone 2a, nitropentafluoroacetone 2b, perfluorocyclohexanone 2c, trifluoropyruvic acid methyl ester 3, as well as the relatively recently described methyl difluorochlorpyruvate 4, were used as electrophilic agents [12].
Heating at 120°C for 1 hour in glacial acetic acid of equimolar amounts of 1 and hexafluoroacetone has been shown to result in the formation with a high yield of the product from C‑oxyalkylation of 5a (see Scheme 1).
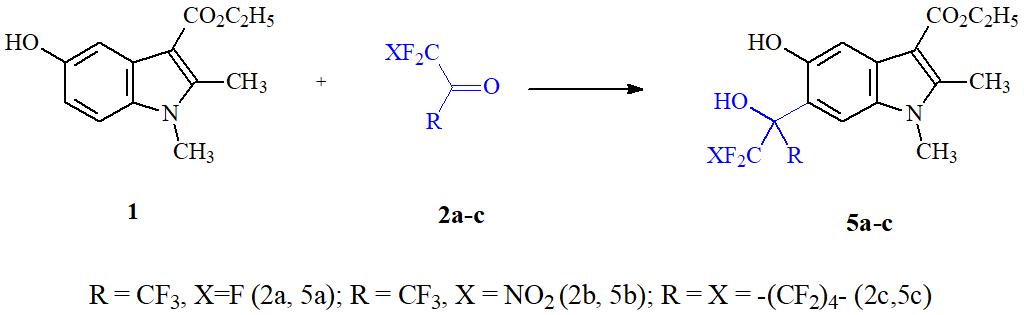
Scheme 1.
The reaction is carried out regioselectively in position 6 of oxyindole 1 with complete conversion of the latter. Under the same conditions nitropentafluoroacetone 2b reacts with compound 1 to form product 5b with 80% yield. Similarly, to hexafluoroacetone, the poorly studied in the oxyalkylation reactions 2,2,3,3,4,4,5,5,6,6 - dodecafluorocyclohexanone 2c reacts with dimecarbine, forming highly fluorinated oxyindole (5b) with a yield of 74%.
In 1Н NMR spectrum of newly synthesized compounds 5a-c significant broadening of protons signals of phenol and polyfluoropropanol OH-groups is observed. It appears that this is associated with intensive proton exchange between their oxygen atoms.
Under similar conditions, reaction is carried out with dicamcarbine with ketoesters 3 and 4 (see Scheme 2).
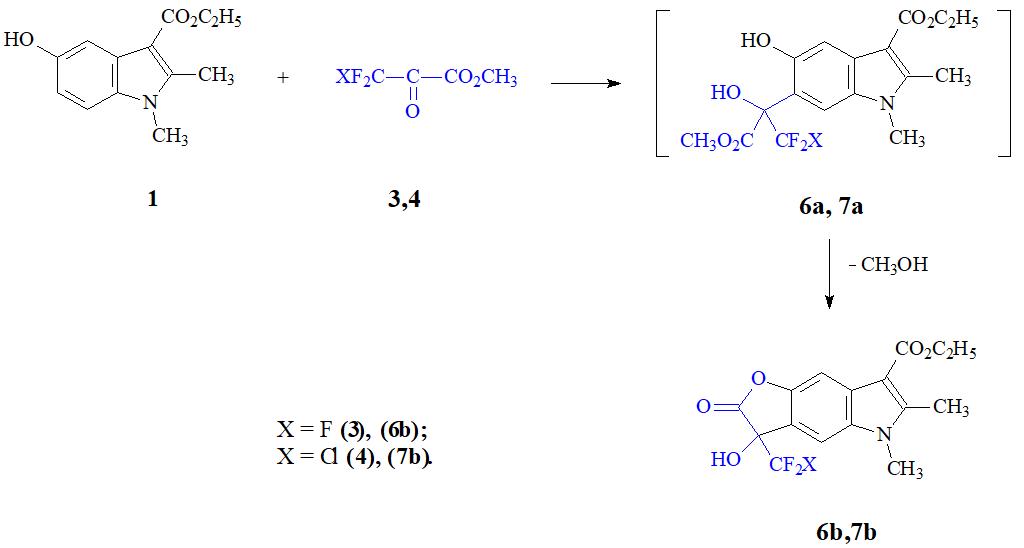
Scheme 2.
Under these conditions, intermediate C-6-oxyalkylation products 6a, 7a are cyclized to the corresponding lactones 6b, and 7b by yield 86.7% and 75%, respectively. It should be noted that this is the first example of using the ketoester 4 in the C-oxyalkylation reaction of similar type hetararomatic compounds.
Dimecarbine has poor solubility and acetic acid was used as a suitable high-boiling polar solvent. A relatively high reaction temperature is most necessary for better solubility of the starting 5-OI rather than for activating the reagents. It appears that C-oxyalkylation 1 can be carried out at a significantly lower temperature.
Therefore, in the example of dicamelbine it is shown that polyfluorocarbonyl compounds 2a‑c and ketoesters 3, 4 are convenient reagents for single-step addition of alpha-hydroxypolyfluoroalkyl substituent to 6 position of 5-OI, including compounds having known biological activity (antibacterial, antiviral, hypotensive, еtс.).
It appears that this method of fluoroalkyl modification can be applicable to other multi-numerical Nenitzescu reaction products, including annelated oxyindoles of a more complex structure.
Presence of chelate function in the compounds 2a-c enables obtaining different types of annelated 1,3-dioxaphosphorinanes [13], which significantly expands synthetic possibilities based on the obtained fluorine-containing oxyindoles 5a-c.
Experimental part
1Н and 19F NMR spectra of the obtained compounds 5a, 5c, 6b and 7b are taken in DMSO-d6 and CDCl3 on a Bruker Avance 300 device (300 and 282 MHz, respectively), compound 5b on Bruker Avance 400 (400 and 376 MHz, respectively). Chemical shifts in 1Н NMR spectra are given in the scale δ (ppm) relative to TMS (internal standard), in NMR 19F spectra relative to CCl3F (external standard). The spin-spin interaction constants are given in Hz. The elemental analysis is performed in the microanalysis laboratory of INEOS RAS. Reactions are monitored by TLC method on Merck plates (silica gel 60 F254, 0.25 mm). The RF values of the synthesized compounds are determined in the acetone-СCl4 = 1:2 system.
5-Hydroxy-1,2-dimethyl-6-(2,2,2-trifluoro-1-hydroxy-1-trifluoromethylethyl)-1H-indole-3-yl carboxylic acid ethyl ester (5a)
In a glass ampoule were placed 2.33 g (10 mmol) 1 and 6 ml glacial acetic acid. The ampoule is then cooled to -78°C and condensed therein 2.00 g (12 mmol) of hexafluoroacetone 2, sealed and heated in an oil bath for 1 hour at 120°C. Ampoule is cooled to -78°C, opened, crystalline precipitate is filtered, washed with acetic acid, then benzene and dried in vacuum. Obtained: 3.60 g of compound 5a in the form of colorless crystals, yield 90.2%, m.p. 252 -254°C (acetic acid), Rf = 0.6 (acetone-CCl4 = 1:2).
1Н NMR spectrum (DMSO-d6, δ, ppm, J/Hz): 10,57 (br. s, 1Н, ОН); 8,83 (br. s, 1Н, ОН); 7,56 (s, 1Н, Ar); 7,54 (s, 1Н, Ar); 4,27 (q, 2Н, OCH2CH3, 3JН-Н=7); 3,68 (s, 3Н, NCH3), 2,70 (s, 3Н, CH3), 1,36 (t, 3Н, OCH2CH3, 3JН-Н=7).
19F NMR spectrum (DMSO -d6, δ, ppm, J/Hz): -73,48.
Found (%): С, 47,86; Н, 3,76, N, 3,96. С16H15F6NO4.
Calculated (%): С, 48,13; Н, 3,79, F 3,51.
6-[1-Difluoromethyl)-2,2,2-trifluoro-1-hydroxyethyl]-5-hydroxy-1,2-dimethyl-1H-indole-3-yl carboxylic acid ethyl ester (5b)
In a glass ampoule were placed 1.16 g (5 mmol) 1, 4 ml of glacial acetic acid and 1.0 g (5.5 mmol) of nitropentafluoroacetone. The ampoule was cooled to -78°C, sealed and heated in an oil bath for 1 hour at 120°C. After cooling to -78°C, the ampoule was opened, its contents were carried out into a flask and evaporated on a rotary evaporator. Solid residue was crystallized from nitromethane. Obtained: 1.7 g of white crystalline compound 5b. Yield is 80%, m.p. is 235°C (subl.) (nitromethane), Rf = 0.51 (acetone-CCl4 = 1:2).
1Н NMR spectrum (СDСl3, δ, ppm, J/Hz): 10,15 (br. s, 1Н, ОН); 8,87 (br. s, 1Н, ОН); 7,13 (с, 1Н, Ar); 7,43 (s, 1Н, Ar); 4,37 (q, 2Н, OCH2CH3, 3JН-Н=7); 3,73 (s, 3Н, NCH3), 2,74 (s, 3Н, CH3), 1,46 (t, 3Н, OCH2CH3, 3JН-Н=7).
19F NMR spectrum (СDСl3, δ, ppm, J/Hz): -73,66 (t, 3F, CF3, 3JF-F=5,6); -90,17 (qq, 1F, CF2NO2, 2JF-F=165, 3JF-F=7,5); -94,16 (br. qq, 1F, CF2NO2, 2JF-F=165, 3JF-F=7,5).
Found (%): С, 44,80; Н, 3,64; N, 6,49. С16H15F5N2O6.
Calculated (%): С, 45,08; Н, 3,55; N 6,57.
6-(2,2,3,3,4,4,5,5,6,6-Decafluoro-1-hydroxycyclohexyl)-5-hydroxy-1,2-dimethyl-1H-indole-3-carboxylic acid ethyl ester (5c)
In a glass ampoule were placed 1.16 g (5 mmol) of 1, 4 ml of glacial acetic acid and 1.5 g (5.4 mmol) of perfluorocyclohexanone. Then it was cooled to -78°C, sealed and heated in an oil bath for 1 hour at 120°C. After cooling to -78°C, the ampoule was opened, its contents were evaporated on a rotary evaporator, the solid residue was crystallized from nitromethane. Obtained: 1.9 g of white crystalline compound 5c, outlet 74%, m.p. 270°C (subl.) (nitromethane), Rf = 0.56 (acetone-CCl4= 1: 2).
1Н NMR spectrum (DMSO-d6, δ, ppm, J/Hz): 11,51 (br. s, 1Н, ОН); 9,65 (br. s, 1Н, ОН); 7,66 (s, 1Н, Ar); 7,57 (s, 1Н, Ar); 4,28 (q, 2Н, OCH2CH3, 3JН-Н=7); 3,67 (s, 3Н, NCH3); 2,70 (s, 3Н, CH3); 1,36 (t, 3Н, OCH2CH3, 3JН-Н=7).
19F NMR spectrum (DMSO-d6, δ, ppm, J/Hz): - 34,85 (dd, 2F, CF2, 2JF-F=282); 38,99 (dd, 2F, CF2, 2JF-F=271); - 42,58 (dd, 1F, CF2, 2JF-F=267); - 54,44 (dd, 2F, CF2, 2JF-F=282); 58,28 (dd, 2F, CF2, 2JF-F=271); 62,50 (dd, 1F, CF2, 2JF-F=282).
Found (%): C, 44,51; Н, 3,15, F 36,81. С19H15F10NO4.
Calculated (%): C, 44,63; Н, 2,96, F 37,16.
3-Hydroxy-5,6-dimethyl-2-oxo-3-trifluoromethyl-3,5-dihydro-2H-1-oxa-5-aza-s-indacene-7-yl carboxylic acid ethyl ester (6b)
Into a glass flask equipped with a reflux condenser with a calcium chloride tube and a magnetic stirrer with heating were placed 2.33 g (10 mmol) 1, 6 ml of glacial acetic acid, 1.8 g (12 mmol) 3 and refluxed for 1 h at 120°C. Then reaction mass was cooled to 20°C, precipitate is filtered, washed with acetic acid, then benzene and dried in vacuum. Obtained: 3.1 g of compound (6b) in the form of colorless crystals, yield 86.8%, Rf = 0.48 (acetone- CCl4= 1:2), m.p. 250-252°C (ethanol).
1Н NMR spectrum (DMSO -d6, δ, ppm, J/Hz): 8,33 (s, 1 Н, ОH); 7,83 (br. s, 1 Н, Ar); 7,76 (s, 1 Н, Ar); 4,30 (q, 2 Н, ОСН2СН3, 3Jн-н = 7,0); 3,79 (s, 3 Н, NСН3); 2,74 (s, 3 Н, СН3); 1,36 (t, 3 Н, ОСН2СН3, 3Jн-н = 7,0);
19F NMR spectrum (DMSO -d6, δ, ppm): -77,5 (s, 3F, СF3).
Found (%): С, 53,83; Н, 4,08; N, 3,96. С16H14F3NO5.
Calculated (%): С, 53,79; Н, 3,95, N 3,92.
3-(Difluoromethyl)-3-hydroxy-5,6-dimethyl-2-oxo-3-trifluoromethyl-3,5-dihydro-2H-oxa-5-aza-s-indacene-7-yl carboxylic acid ethyl ester (7b)
Into a glass flask equipped with a reflux condenser with a calcium chloride tube and a magnetic stirrer with heating were placed 1.17 g (5 mmol) 1, 3 ml of glacial acetic acid, 1 g (5.8 mmol) 3 and refluxed for 4 hours. Then the reaction mass was evaporated on a rotary evaporator, the resulting residue was crystallized from nitromethane. Obtained: 1.4 g of white substance 7b, 75% yield, Rf = 0.49 (acetone- CCl4= 1:2), m.p. 247 -248°C (nitromethane).
1Н NMR spectrum (DMSO -d6, δ, ppm, J/Hz): 8,41 (s, 1 Н, ОH); 7,81 (br. s, 1 Н, Ar); 7,75 (s, 1 Н, Ar); 4,30 (q, 2 Н, ОСН2СН3, 3Jн-н = 7,0); 3,78 (s, 3 Н, NСН3); 2,74 (s, 3 Н, СН3); 1,35 (t, 3 Н, ОСН2СН3, 3Jн-н=7,0);
19F NMR spectrum (DMSO -d6, δ, ppm): -66,53 (d, 1F, СF2Cl, -65,97 (d, 1F, СF2Cl, 2JF-F=163,8);
Found (%): С, 51,27; Н, 3,87; N, 3,88. С16H14СlF2NO5.
Calculated (%): C, 51,42; Н 3,78; N, 3,75.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Contract/agreement No. 075-03-2023-642). NMR spectral analysis of synthesized compounds were performed with support of Russian Science Foundation (grant No. 22-23-00559) employing the equipment of Center for molecular composition studies of INEOS RAS.
The authors express their gratitude to Dr. Osipov S. N. for the methyl ester of difluorochloropyruvic acid presented for research.
References
- S. N. Young, J. Psychiatry Neurosci, 2007, 32(6), 394-399.
- S. Benmansour, M. Cecchi, D. A. Morilak, G. A. Gerhardt, M. A. Javors, G. G. Gould, A. Frazer, J. of Neuroscience, 1, 1999, 19(23), 10494-10501.
- L. A. Newman, M. T. Walker, R. L. Brown, T. W. Cronin, P. R. Robinson, Biochemistry, 2003, 42(44), 12734-12738.
- M. D. Mashkovsky, Medicines (a manual on pharmacotherapy for doctors), 1978, Ed. Medicine, 1344 p.
- WO9008135 (1990).
- F. A. Trofimov, N. G. Tishkova, S. A. Zotova, A. N. Grinev, Khim. Pharm. Zh., 1993, 1, 70-71.
- (a) Grinev, A. N. et al., Pharmaceutical Chemistry Journal, 1970; 1, 25-29; (b) Kurilo G. N.; Rostova N. I.; Cherkasova A. A., Turchin, K. F, Alekseeva L. M., Grinev A. N., Chemistry of Heterocyclic Compounds, 1980, 1043–1047; (c) Trofimov F. A., Tsyshkova N. G., Zotova S. A., Grinev A. N., Pharmaceutical Chemistry Journal, 1993, 27, 1, 75-76.
- M. Novak, K. J. Kayser, M. E. Brooks, J. Org. Chem., 1998, 63, 5489-5496.
- Ishikawa N., Fluorine compounds. Synthesis and application, M.: Mir. 1990. 407 p.
- 10 (a) V. M. Muzalevskiy, Z. A. Sizova, V. G. Nenajdenko, Molecules, 2021, 26(16), 5084; (b) D. V. Vorobyeva, T P. Vasilyeva, S. N. Osipov, Russ. Chem. Bull., 2022, 71, 1949; (c) Sigan A. L., Volkonskii A. Yu, Kagramanov N. D., Guseva E. V., Chkanikov N. D., Fluorine notes, 2023, 4(149), 1-2.
- (a) Dyachenko V. I., Galakhov M. V., Kolomiets A. F., Fokin A. V., Bull. Acad. Sci. USSR, Div. Chem. Sci (Engl. Transl.), 1989, 38, 4.2, 831-836; (b) Dyachenko, V. I., Kolomiets, A. F., Fokin A. V., Bull. Acad. Sci. USSR, Div. Chem. Sci (Engl. Transl.), 1987, 36, 995-1000; (c) Dyachenko V. I., Kolomets A. F., Fokin, A. V., Bull. Acad. Sci. USSR, Div. Chem. Sci (Engl. Transl.), 1987, 36, 12, 2646-2648; (d) Dyachenko V. I., Galakhov M. V., Kolomiets A. F., Fokin A. V., Bull. Acad. Sci. USSR, Div. Chem. Sci (Engl. Transl.), 1989, 38, 12, 2550-2553;
- S. N. Osipov, A. S. Golubev, N. Sewald, T. Michel, A. F. Kolomiets, A. V. Fokin, K. Burger., J. Org. Chem., 1996, 61, 21, 7521–7528.
- (a) E. E. Nifant’ev, T. S. Kukhareva, V. I. Dyachenko, A. F. Kolomiets, N. S. Magomedova, V. K. Bel’skii and L. K. Vasyanina, Phosphorus Sulfur Silicon Relat. Elem., 1994, 92, 29-38; (b) V. I. Dyachenko, A. A. Korlyukov, Mendeleev Commun., 2019, 29, 89–90.
ARTICLE INFO
Received 18 October 2023
Accepted 27 October 2023
Available online October 2023
Recommended for publication by PhD O.V. Bryzgalova
eLIBRARY Document Number (EDN) CVJTMO

Fluorine Notes, 2023, 150, 5-6
