Received: May 2024
DOI 10.17677/fn20714807.2024.03.01
Fluorine Notes, 2024, 154, 1-2
IONIC SERIES OF HEXAKIS (TRIFLUOROMETHYLTHIO)- AND HEXAKIS (TRIFLUOROMETHYLSELENO) BENZENES AND FLUOROBENZENES WITH PERFLUOROALKYL SUBSTITUENTS
N. D. Kagramanov, E.I. Mysov
A. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991, GSP-1, Moscow,
B-334, Vavilov St. 28
e-mail: ndkagram@gmail.com
Abstract: The present report is a continuation of the earlier studies by studying the ionic series of benzene, 1,3,5,7-cyclooctatetraene, [18]-annulene, perfluorobenzene, hexamethylbenzene and hexakis (trifluoromethyl) benzene. The aim of the invention is to explain how the sulfur and selenium atoms change the fragmentation of benzenes with trifluoromethyl substituents.
Keywords: mass spectra and ionic series C12F18S6, C12F18Se6, C12F18, C10F14, C16F26.
Introduction
According to the L.M. Yagupolskii, N.V. Kondratenko, V.P. Sambur [1] invention, F3CSCu has been shown to be a convenient reagent for replacing halide atoms in aryl- and heterohalides with a trifluoromethylthio- group. Hexasubstituted benzenes with perfluorinated groups separated from benzene ring by sulfur and selenium atoms were synthesized for the first time. The structures of compounds C12F18S6 and C12F18Se6 have been confirmed by NMR spectra 19F, IR spectra, UV spectra and mass spectra [2]. Mass spectra were obtained by Senior researcher of INEOS RAS USSR organofluoric compounds laboratory E.I. Mysov (head of a laboratory – acad. I.L.Knuniants), on a device MX-1310, with an ionizing voltage of 18 eV. The spectra contained only molecular ions, since the format of the short message excludes the possibility of complete mass spectra publication of two polyisotopic compounds with masses of 678 and 966 amu. Since the samples were preserved, their mass spectra were repeated at a standard voltage of 70 eV on a Finnigan Polaris Q mass spectrometer. Thus, the present message is a continuation of studies previously performed on the study of the ionic series of benzene, 1,3,5,7-cyclooctatetraene, [18]-annulene, perfluorobenzene [3], hexamethylbenzene and hexakis(trifluoromethyl)benzene [4]. The aim of the invention is to identify the sulfur and selenium atoms effect on changing the fragmentation of benzenes with trifluoromethyl substituents. Heavy atoms of sulfur and selenium themselves are able to form six-member cycles S6 and Se6. For this reason, at the fragmentation of C12F18S6 and C12F18Se6 is likely to occur in successive emissions of six F and CF2 atoms with the formation of stable cation-radicals +C6S6 and +C6Se6 with novel sulfur-sulfur and selenium-selenium bonds, and then the separation of sulfur and selenium atoms begins. In contrast to fragmentation of hexakis(trifluoromethylthio)- and hexakis(trifluoromethylseleno)benzenes, in ionic series of two isomers of C12F18, unprotected by heavy atoms S and Se along with primary separation one fluorine atom primary separation there is a primary synchronous release of two, as well as three fluorine atoms with formation of new bonds and three main ionic series. Apparently, sulfur and selenium atoms, more specifically, six bonds with CF3 groups and six bonds with a C6 cycle take part of the excitation energy +M, which is necessary for primary synchronous detachments of two or three fluorine atoms. The perfluoroalkyl ionic series C12F18S6 and C12F18Se6 differ. In spectrum C12F18Se6 intensive (46%) peak +CF3 is formed, while in spectrum C12F18S6 there is a series of ions: +C3F7 (4%), +C2F5 (0.4%), +CF3 (4%). One of the ionic series C12F18S6 after successive separation of three radicals .CF3 branches, since there is a break of the C6 cycle with a C3S3 emission and the formation of an ion +C3S3(CF3)3 m/z 339 (0.4%).
As a result of the following detachments: C3, two fluorine atoms and three sulfur atoms, there is a rearrangement ion +C3F7 with m/z 169 (4%).
Experimental part
The electrons ionization mass spectra are recorded on a chromatography-mass spectrometer «Finnigan Polaris Q» ion trap, a range of 14-1000 Da, energy of 70 eV, and direct input of DIP (heating at a rate of 10°/min).
Mass spectra and ionic series of hexakis (trifluoromethylthio)-and (trifluoromethylseleno) benzenes
Figures 1 and 2 shows the mass spectrum and the ionic series of hexakis(trifluoromethylthio)benzene.
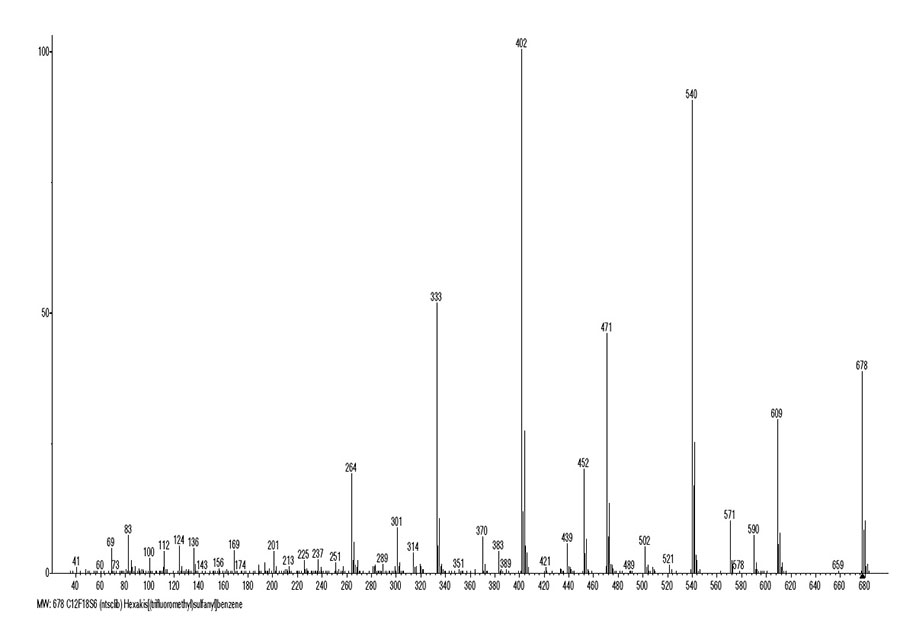
Figure 1. The mass spectrum of hexakis (trifluoromethylthio) benzene C12F18S6 MW:678.
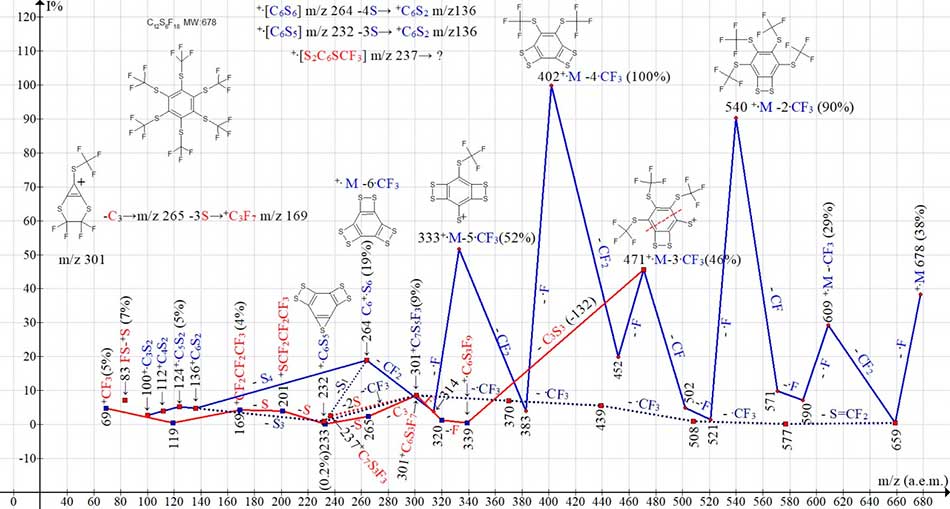
Figure 2. The ionic series of hexakis (trifluoromethylthio) benzene C12F18S6 MW:678 mass spectrum.
The mass spectrum of hexakis(trifluoromethylthio)benzene (Figures 1 and 2) consists of one ion series with intense fragmentation peaks (marked with blue color) and a subset with less intense peaks (marked with a dotted line blue). In the main series, successive separation: F, CF2, (F, F, CF)2, (F, CF2)3 lead to ion +C6S6 with m/z 264 (19%), fragmented first with separation S4, and then with the separation of three carbon atoms. The main series branches at least twice. The first branching of the main series (marked with a dotted line of blue color) starts with the detachment of S=CF2 from the ion with m/z 659, and then four consecutive emissions of CF3, with the formation of an ion +C6S5CF3 with m/z 301 (9%). This ion ejects the latter group CF3 (301-69 = m/z 232) and three sulfur atoms, turning into ion +C6S2 with m/z 136.
Another branching of the main ionic series (marked with red color) is the alternative fragmentation result of the ion with m/z 471, terminating in the rearrangement ion +C3F7 formation. After detachment of the three radicals, the resulting asymmetric ion with m/z 471 fragments with the interruption of the benzene cycle in half and the separation of the C3S3 molecule to form an ion +C3(SCF3)3 with m/z 339 (0.4%). The ion with m/z 339 loses two fluorine atoms, turning into an ion +C6S3F7 with m/z 301 (8.6%). It should be noted that ions with a nominal mass m/z 301 arising in two different branches of the main series have different structures: +C6S5CF3 and +C6S3F7. Next, the ion +C6S3F7 loses C3 (-36) and, as a result of successive detachments of three sulfur atoms, turns into a rearrangement ion +C3F7 with m/z 169 (4%).
Figures 3 and 4 shows the mass spectrum and the ionic series of hexakis (trifluoromethylseleno) benzene C12F18Se6.
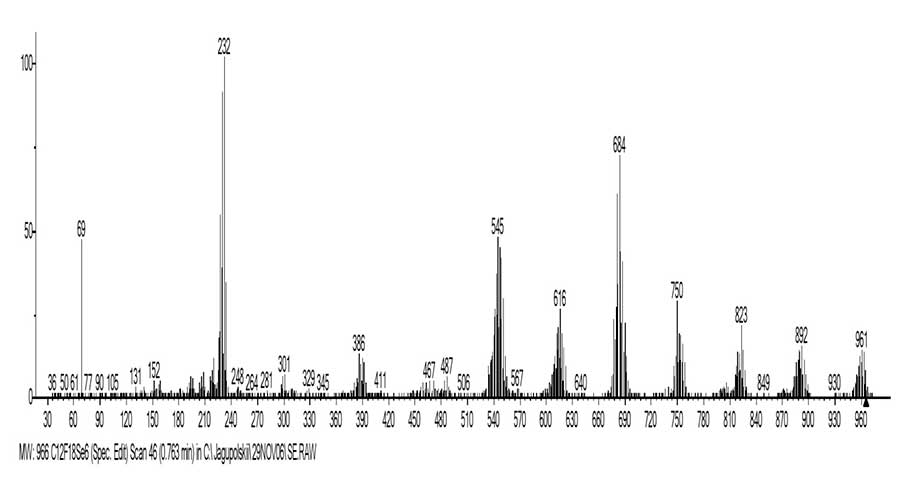
Figure 3. The mass spectrum of hexakis (trifluoromethylseleno) benzene C12F18Se6 MW:966.
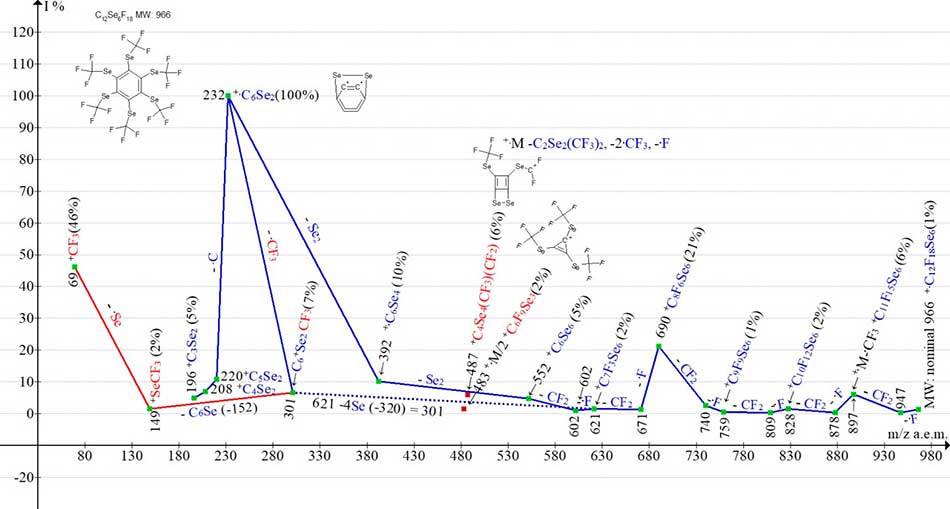
Figure 4. The ionic series of hexakis (trifluoromethylseleno) benzene C12F18Se6 MW:966 mass spectrum.
The mass spectrum of C12F18Se6 hexakis (trifluoromethylseleno) benzene (Figures 3 and 4) consists of one ion series (marked with blue color). It branches at least three times, since in the spectrum along with ion m/z 621 C6Se6CF3 ions arise: with m/z 483 (m/2), as well as ion c m/z 487 (6%) +M-C2Se2(CF3)2, -F. In addition to the molecular ion, with a nominal mass of m/z 966 (1.4%), the series comprises six stacks of isotopic peaks. The peaks correspond to six consecutive separation of the atom of F and CF2 (Σ CF3) to form +C6Se6 with nominal mass with m/z 552 (5%). In contrast to the mass spectrum of C12F18S6, in the basic ionic series of which more energy-intensive successive emissions occur twice (‑F, ‑F, -CF), in the ionic series C12F18Se6 this does not occur. Their spectra differs by the fact that the base peak in the spectrum of C12F18Se6 is the peak +C6Se2 with m/z 232 (also in the spectrum Se6), while in the spectrum C12F18S6 the intensity of the peak +C6S2 with m/z 136 is only 5%. The base peak in the C12F18S6 spectrum is the peak +C8S6F6 with m/z 402 (+M -4CF3), whereas in the C12F18Se6 spectrum the intensity of this peak with a nominal mass m/z 690 is 21%. Another difference in their fragmentation is that, unlike the C12F18S6 spectrum, where after separation of the three CF3 radicals, the C9F15S6 cycle decay on C3S3 and +C3S3(CF3)3 occurs, this does not occur in the C12F18Se6 spectrum. In the spectrum C12F18Se6, a single intense ion peak +CF3 (46%) occurs, whereas in the +C3S3(CF3)3 spectrum, a series of low-intensity peaks of perfluoroalkyl ions are observed: +C3F7, +C2F5 and +CF3.
Another difference in the mass spectra of C12F18S6 and C12F18Se6 is the fact that only in the spectrum C12F18Se6 an ion peak M/2 with m/z 483 (2%) is formed, whereas in the spectrum C12F18S6 the peak intensity M/2 c m/z 339 (0.4%) is formed.
Mass spectra and ionic series of fluorobenzenes with perfluoroalkyl substituents
Figures 5 and 6 shows the mass spectrum and the ionic series of hexakis(trifluoromethyl)benzene C12F18.
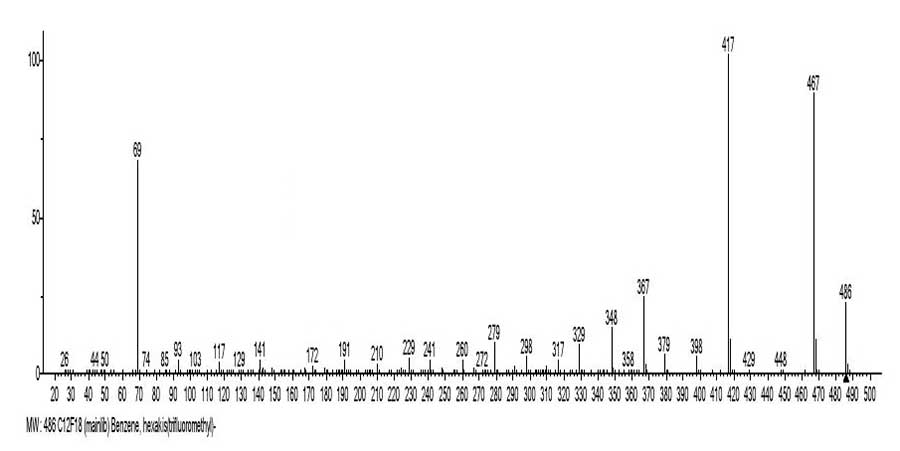
Figure 5. The mass spectrum of hexakis (trifluoromethyl) benzene C12F18 MW:486
NIST #: 151287ID #: 302846 DB: mainlib.
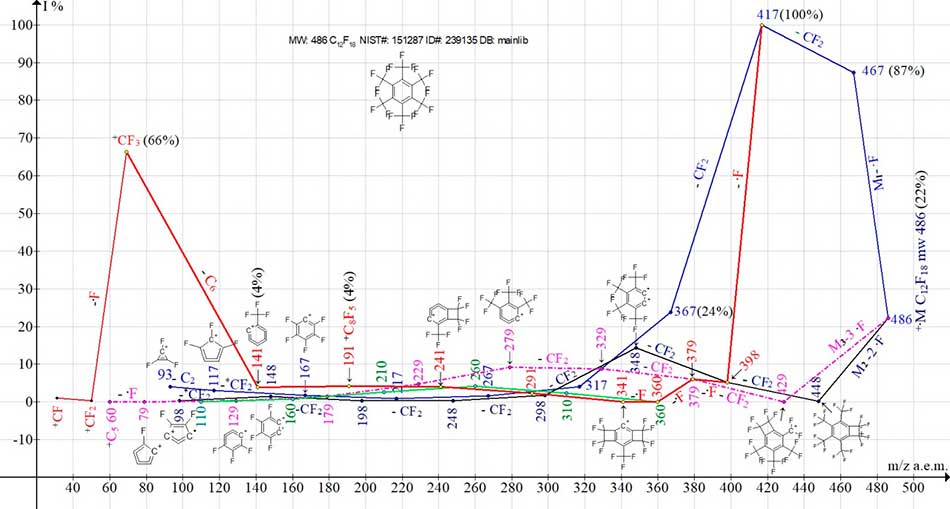
Figure 6. The ionic series of hexakis (trifluoromethyl) benzene C12F18 MW:486 mass spectrum.
NIST#: 151287 ID#: 302846 DB: mainlib.
Mass spectrum of hexakis (trifluoromethyl) benzene (Figure 5) includes three ionic series M1-M3 (Figure 6) and two branches of series M1 [4].
Three series M1-M3 of spectrum С12F18 is a result of molecular cation-radicals +M1-3 fragmentation, three times differing in energy of fluorine atoms primary separation.
This is the separation of one fluorine atom +.M1-F m/z 467, synchronous separation of two atoms +.M2-2F m/z 448 and synchronous separation of three fluorine atoms +.M3-3F m/z 429. As a result of the subsequent CF2 (-50) regular emissions, three ionic series are formed, with the last significant masses digits: 7, 8 and 9. It should be noted that similar synchronous primary detachments of one, two and three radicals occur and in the n-alkanes and n-perfluoroalkanes [5] fragmentation, as well as polyoxaperfluoroalkanes and polyoxaalkyl halides [6].
Separation of fluorine atom from one of terminal CF3 groups of perfluorotertbutylamine (series M-F) is also accompanied by two more fluorine atoms synchronous detachment from two other groups CF3, so that first peak of this series of spectrum is peak of ion M-3F m/z 614 (4%) [7]. The synchronous primary detachments of three fluorine atoms also take place in the perfluorocyclohexane fragmentation [5].
Figures 5 and 6 shows the mass spectrum and the ionic series of 1,2,3,4-tetrafluoro-5,6-bis(heptafluoropropyl) benzene C12F18 MW:486.
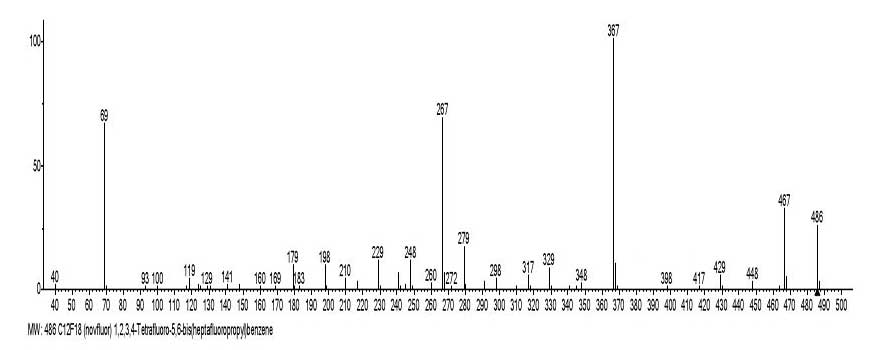
Figure 7. The mass spectrum of 1,2,3,4-tetrafluoro-5,6-bis(heptafluoropropyl) benzene
C12F18 MW:486. NIST #: 110830 ID#: 298066 DB: mainlib.
.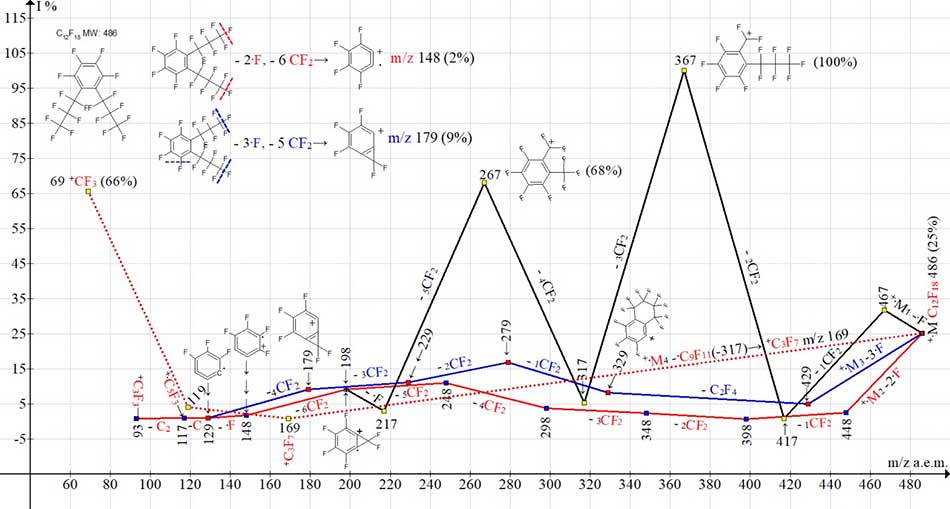
Figure 8. Four ionic series of 1,2,3,4-tetrafluoro-5,6-bis(heptafluoropropyl)benzene
C12F18 MW:486 mass spectrum. NIST#: 110830 ID#: 298066 DB: mainlib.
In the ionic series of two isomers of C12F18, symmetrical (Figure 6) and asymmetric (Figure 8), unprotected by sulfur and selenium heavy atoms, primary synchronous breaks of three (+M3 -3F), two (+M2 -2F), and one fluorine atom (+M1 -1F) occur.
In the ionic series of hexasubstituted benzenes C12F18S6 and C12F18Se6 with trifluoromethyl groups separated from the benzene ring by sulfur and selenium atoms, the primary synchronous detachments of three and two fluorine atoms do not occur. They are fragmenting by six consecutive emissions of F и CF2 atoms to form stable cation-radicals +C6S6 and +C6Se6. Subsequent separations of sulfur and selenium atoms is then initiated. The sulfur and selenium atoms, more specifically six of their bonds with the CF3 groups and six bonds with the C6 cycle appear to take part of the excitation energy +M, which is necessary for synchronous primary detachments of two, as well as three fluorine atoms.
In mass spectra of fluorobenzenes with perfluoroethyl substitutes isolated from each other by two fluorine atoms, primary synchronous separations of two and three fluorine atoms does not occur. Figures 9 and 10 shows the mass spectrum and ionic series of 1,2,3,4-tetrafluoro-3,6-bis (pentafluoroethyl) benzene.
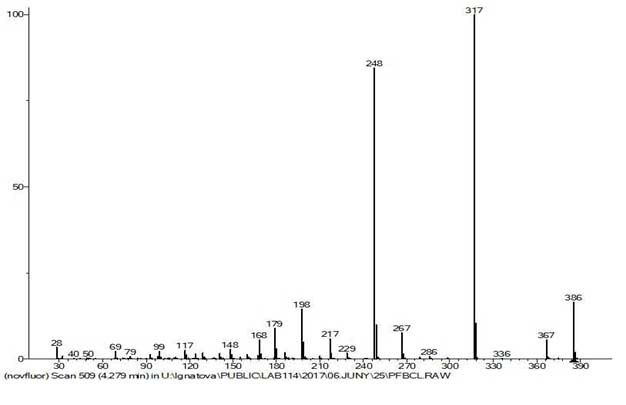
Figure 9. The mass spectrum of 1,2,4,5-tetrafluoro-3,6-bis(pentafluoroethyl) benzene
C10F14 MW: 386, P&M Invest.
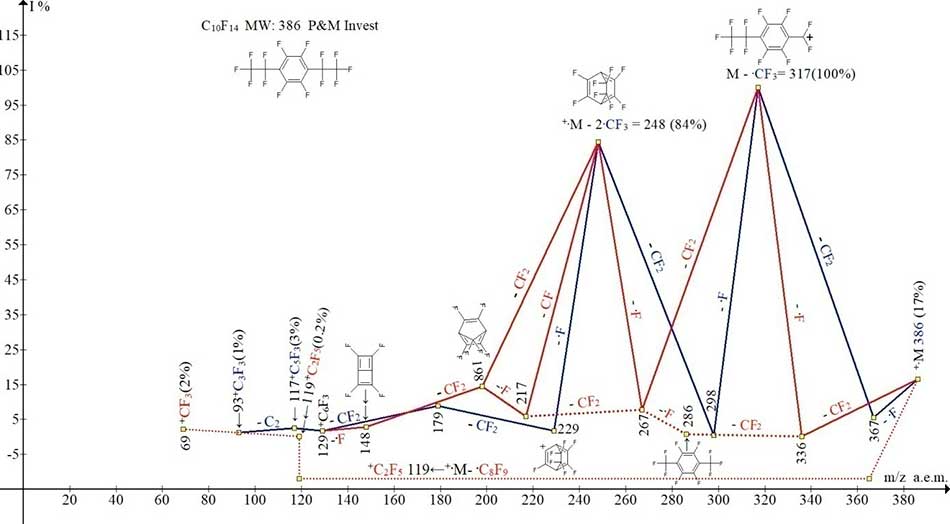
Figure 10. The ionic series of 1,2,3,4-tetrafluoro-3,6-bis(pentafluoroethyl) benzene
C10F14 MW: 386, P&M Invest.
In the mass spectrum of 1,2,3,4-tetrafluoro-3,6-bis(pentafluoroethyl)benzene (Figures 9 and 10) with pentafluoroethyl substitutes, located in para-position relative to each other and separated by two fluorine atoms, occurs only successive separation of the atoms of F and CF2, as well as CF2 and F. But there are no primary synchronous detachments of three and two fluorine atoms, since both perfluoroethyl groups are isolated from each other by two fluorine atoms.
An interesting examples are that two perfluoroisopropyl benzene substituents in the fourth and sixth positions and one trifluoromethyl substituent in the fifth position are fragmented by primary synchronous separation of one, two and three fluorine atoms are mass spectrum (Figure 11) and ionic series (Figure 12) of 1,3-difluoro-2,4,6-tris(perfluoroisopropyl)-5-trifluoromethyl benzene.
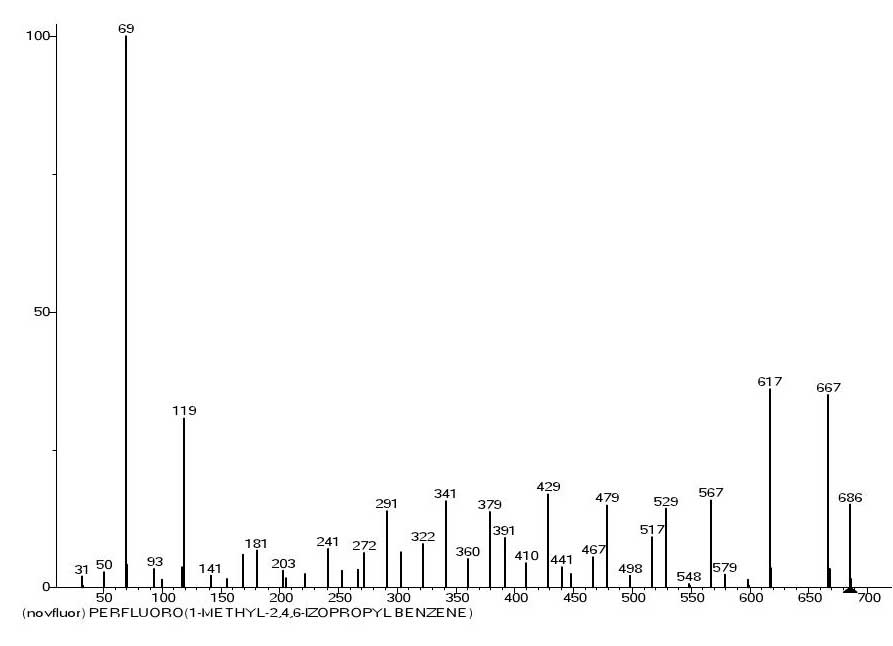
Figure 11. The mass spectrum of 1,3-difluoro-2,4,6-tris(perfluoroisopropyl)-5-trifluoromethyl benzene C16F26 MW: 686, INEOS RAS.
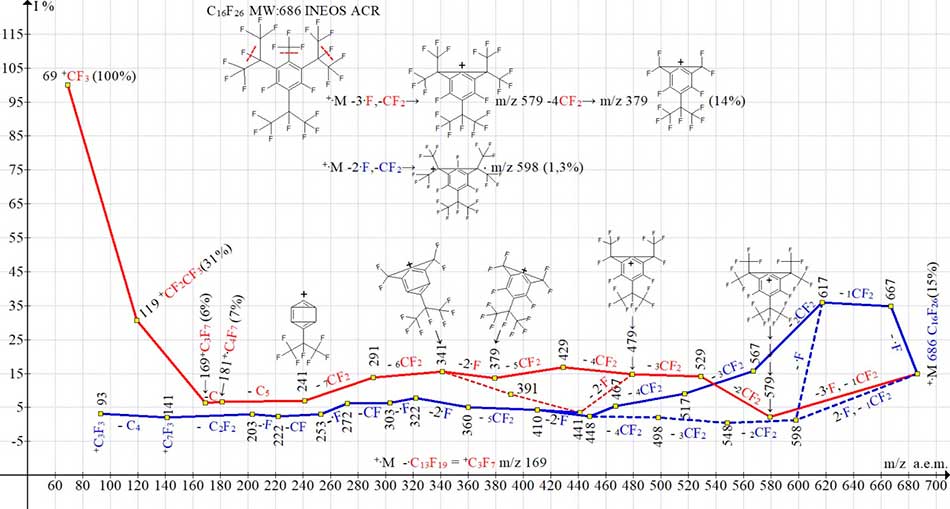
Figure 12. Three ionic series of 1,3-difluoro-2,4,6-tris(perfluoroisopropyl)-5-trifluoromethylbenzene C16F26 MW: 686.
As a result of primary synchronous detachments of one fluorine atom, two fluorine atoms and CF2 group, as well as three fluorine atoms and CF2 group, there are three ionic series branching and changing into each other.
The regular fragment group CF2 (-107 and -88) addition to three (-57) and two fluorine atoms (-38) detachments does not violate the separation rules of three and two radicals, since this is the result of peaks +M-3F and +M-2F low intensity and higher intensities of peaks with additional separation of the regular fragment group CF2. Similar primary separations +M-3F, -CF2 (-107) also occur in the of perfluoro-2,4-dimethyl-3-ethylpentane-C9F20 and perfluoroeicosane-C20F42 [5] fragmentation.
In the case of synchronous separation of three as well as two fluorine atoms from two or one of perfluoroisopropyl substituents (Figure 12), a trifluoromethyl substituent is also involved in fragmentation. Fragmentation of C16F26 is completed by two ionic series (Figure 12) marked with blue and red. In the series marked with red color arising as a result of the primary synchronous separation of three fluorine atoms and CF2 group, two perfluoroisopropyl substituents are involved, as well as a trifluoromethyl substituent. The perfluoroisopropyl substituent in the second position, separated from other substituents by fluorine atoms in the first and third positions, is involved in fragmentation only at the final stage, forming a series of perfluoroalkyl ions: +C3F7 m/z 169 (6%), +C2F5 m/z 119 (31%) and +CF3 m/z 69 (100%).
Conclusions
During the ionization of molecules, depending on the number and energies of their electronic bonds, absence or presence of sulfur or selenium atoms chains, molecular ions either acquire three-fold differing excitation energies, or energy, leveled with electronic links, insufficient for three-fold energy difference of primary separations. In the mass spectra of perfluorobenzenes with perfluoroalkyl substituents, synchronous separation of one, two and three fluorine atoms does not occur if the perfluoroalkyl substituents are separated from each other by two fluorine substituents. In contrast to primary synchronous detachments of three fluorine atoms in ionic series of perfluorotributyl amine [7], in 2,4,6-tris(perfluoroalkyl)-1,3,5-triazines mass spectra, primary synchronous separation of three fluorine atoms does not occur [8], since their perfluoroalkyl substituents are separated from each other by three nitrogen atoms. An interesting example of that two perfluoroisopropyl benzene substituents at positions 4 and 6 and one trifluoromethyl substituent at position 5 are fragmented by primary synchronous separation of one, two and three fluorine atoms is a mass spectrum and an ionic series of 1,3-difluoro-2,4,6-tris(perfluoroisopropyl)-5-trifluoromethyl benzene.
In the ionic series of n-alkanes, n-perfluoroalkanes, perfluorocyclohexane and polyoxaperfluoralkanes spectra, the energies of the primary synchronous detachments of the radicals also differ by three times [6].
The specific type of fragmentation is the molecular ions isomerization, typical for cyclic molecules with CH bonds. An examples of molecular ion isomerisation are: six ion series of benzene mass spectrum, arising as a result of its carbon skeleton restructuring, in accordance with five versions of π-conjugates, eight non-aromatic 1,3,5,7-cyclooctatetraene (CH)8 ionic series and fifteen 18-annulene (CH)18 ionic series [3].
Similar values of carbon atoms number and arising ionic series number are the result of the fact that in fragmentation of excited molecular cation-radicals all possible versions of restructuring of their π-conjugates are realized, as well as version of fragmentation with existing conjugations preservation, when only separation of hydrogen atoms takes place. The benzene ionic series, 1,3,5,7-cyclooctatetraene С8H8 and [18]-annulene C18H18 are fragmenting, without branching or intersecting.
Compared to the mass spectrum of benzene, consisting of six ionic series with five options for restructuring π-conjugations, in the spectrum of hexakis (methyl) benzene, the number of ionic series increases to eleven, but only two variants of adjustment are realized: π1-4 and π1-3 [4]. That is, methyl substituents make it difficult to re-tune the π-conjugations, which can result from a four-fold increase in the number of electron bonds and a fifteen-fold increase in the CH3 mass, as compared to the mass of the hydrogen atom.
Replacement in a benzene molecule of one hydrogen atom by a fluorine atom leads to seven new fluorine-containing ionic series, which confirms the presence of π-conjugations not only between CH-groups, but also between CH and CF-groups [3].
The number of hydrocarbon ionic series in the mass spectrum of 1,2-difluorobenzene is reduced to four series. Further hydrogen atoms substitution with fluorine atoms leads not only to a decrease in the number of hydrocarbon ionic series, but also to a decrease in the number of fluorine-containing series [3].
Unlike the six ionic series of benzene, the hexafluorobenzene fragmented to form two ionic series differing by the sequence of separation [3].
The analysis of substituted benzenes ionic series makes it possible to establish fragmentation paths, as well as the presence or absence of the six-membered cycle π-conjugations reconfiguration.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation and was performed employing the equipment of Center for Molecular Composition Studies of INEOS RAS.
References
- L.M. Yagupolskii, N.V. Kondratenko, V.P. Sambur, Synthesis, 1975, 721.
- A.A. Kolomeitsev, N.V. Kondratenko, V.I. Popov, L.M. Yagupolskii, Hexakis-(trifluoromethylthio)- and (trifluoromethylseleno) benzenes. Zh. Org. Khimii, 1983, XIX (12), 2631-2632. (in Russian)
- N. D. Kagramanov, Decay sequences - ion series of mass spectra of benzene, 1,3,5,7-cyclooctatetraene, [18]-annulene, hexafluorobenzene and its isomers, Fluorine Notes, 2022, 3(142), 5-6.
- N. D. Kagramanov, Decay sequences - ion series of mass spectra hexamethylbenzene and hexakis(trifluoromethyl)benzene, Fluorine Notes, 2022, 5(144), 1-2.
- N. D. Kagramanov, Ratios of primary separations in ionic series of mass-spectra of perfluoroalkanes, perfluorocyclohexane, eicosane, cyclotriacontane, containing regular fragment groups (C2H4 or CF2), Fluorine Notes, 2023, 6(151), 1-2.
- N. D. Kagramanov, Synchronous separation of three radicals in ionic series of polyoxaperfluoroalkanes and polyoxaperfluoroalkyl halides mass spectra, Fluorine Notes, 2024, 2(153), 1-2.
- N. D. Kagramanov, Three series ions of perfluorotributylamine mass-spectrum (PFTBA), Fluorine Notes, 2020, 3(130), 1-2.
- N. D. Kagramanov, Decay sequences - ionic series of mass spectra for 2,4,6-tris(perfluoroalkyl)-1,3,5-triazines, Fluorine Notes, 2023, 4(149), 3-4.
ARTICLE INFO
Received 14 May 2024
Accepted 29 May 2024
Available online June 2024
Recommended for publication by PhD M.A. Manaenkova eLIBRARY Document Number (EDN) TJUUTI 
Fluorine Notes, 2024, 154, 1-2
