Received: June 2024
DOI 10.17677/fn20714807.2024.03.03
Fluorine Notes, 2024, 154, 5-6
SYNTHESIS OF 2-ACRYLAMINO-3,3,3-TRIFLUOROPROPIONIC ACID ESTERS
V.I. Dyachenko
A. N. Nesmeyanov Institute of Organoelement Compounds, RAS, Russian Federation, 119334, Moscow, Vavilov St., 28, 1
Fax: (499) 135 5085, e-mail: vic-d.60@mail.ru
Abstract: Initial CF3-containing N-hydroxymethylacrylamides are obtained by reaction of 3,3,3-trifluoropyruvic acid esters with acrylamide. The corresponding chlorine-substituted acrylamides are synthesized during their refluxing in high-yield SnCl2. Reduction of their zinc in acetic acid leads to formation of 2-acrylamino-3,3,3-trifluoropropionic acid esters. The newly synthesized CF3-containing acrylamides are offered as potential monomers.
Keywords: acrylamide, methyl(ethyl)3,3,3-trifluoropyruvate, fluorine-containing monomers, 2-acrylamino-3,3,3-trifluoropropionic acid esters.
Polymers and copolymers based on fluorine-containing acrylamides have a number of valuable specific properties which are not inherent to non-fluorinated analogs [1]. In addition to the appearance of new physical and chemical properties, some of them have the ability to react to an external effect, which is especially desirable in nanotechnology to create sensors, functional materials and microdevices [2-4].
It should be noted that there is currently a very limited amount of commercially available monomers among fluorine-containing acrylamides in order to conduct targeted studies in this area. Consequently, N-(2-fluoroethyl) acrylamide (F1EA), N-(2,2-difluoroethyl) acrylamide (F2EA) and N-(2,2,2-trifluoroethyl) acrylamide (F3EA) (Figure 1) [5] are most commonly used in this direction.
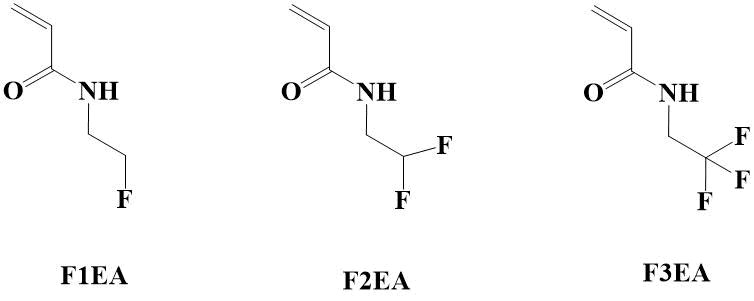
Figure 1. N-(2-fluoroethyl-substituted) acrylamides.
It has been found that F3EA, by virtue of the influence of the electron-withdrawing CF3-group, has a sharply reinforced and red-shifted photoluminescence, which is not characteristic of such small molecules [6]. It is shown that the quantum yield of photoluminescence depends on the conditions and medium (temperature, metal cations), which is promising for use in medical diagnostics.
The promising results of the copolymerization of difluoroacrylamide F2EA with N-(2-hydroxypropyl)methacrylamide are set out in [7]. The water-soluble biocompatible heat-sensitive copolymers and nanogels formed therein have been successfully used for the MRI 19F imaging of angiogenesis and the marking of pancreatic islets.
Very interesting results are obtained as a result of RAFT copolymerization of F1EA, F2EA and F3EA with N,N-dimethylaminoethyl methacrylate. The statistical heat-sensitive copolymers produced on the basis thereof during blowing are capable of repeatedly switching on both oxygen and carbon dioxide [8].
Thus, the above scientific research analysis shows the potential for the synthesis of novel CF3-containing acrylamides as monomers for the production of functional polymer materials.
Generally, N-fluoroalkylacrylamides of formula II are prepared by reacting fluorine-containing alkylamines I with anhydrides (a) or acid chlorides (b) of acrylic acid in the presence of acceptors (NEt3, Py) released in the reaction of acids [9, 10] (Scheme 1).
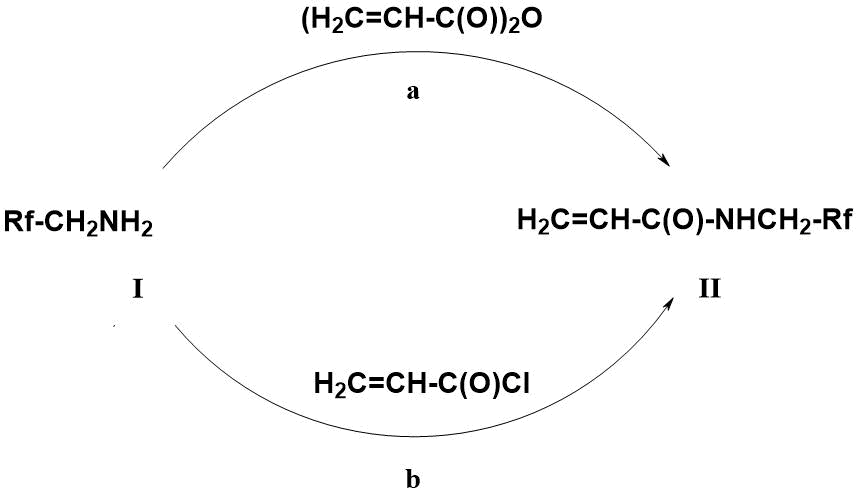
Scheme 1. Preparation of N-fluoroalkyl acrylamides
The unusual approach to the synthesis of the novel compounds presented herein is that the modification of the amine component of acrylamide is carried out directly in the molecule itself (Scheme 3).
It has previously been found that polyfluorocarbonyl compounds (III), unlike their non-fluorinated analogs, readily react with amides (IV) of various aliphatic and aromatic carboxylic acids, forming stable N-amidooxy compounds (V) [11-15] (Scheme 2).
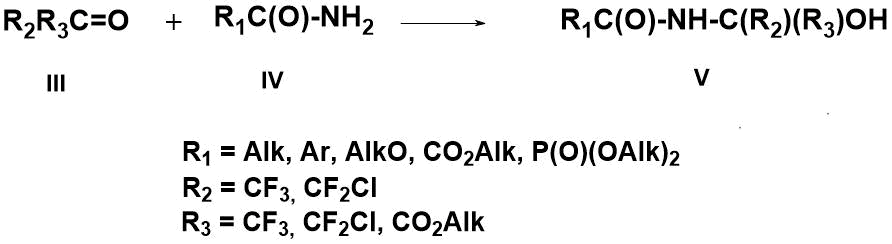
Scheme 2. Preparation of N-amidooxy compounds (V)
It has also been shown that an OH group in compounds of this type under the effect of SOCl2 is readily replaced by chlorine. Halogen in the resulting adducts has sufficiently high mobility and can be easily reducted [15].
At the same time, the above conversions have not been studied for (meth)acrylamides, which are widely used today and are industrially accessible. Indeed, fluoroalkyl-containing acrylamides produced on the basis thereof can be used in the synthesis of functional (co)polymers as described above [2-8].
The aim of the present invention is to obtain novel CF3-containing monomers – methyl 2-acrylamino-2-chloro-, ethyl 2-acrylamino-2-chloro-, methyl 2-acrylamino- and ethyl 2-acryloxyamino-3,3,3-trifluoropropionates 4a,b and 5a,b, respectively (Figure 2).

Figure 2. СF3-containing acrylamides.
Recently, methyl (2a) and ethyl (2b) esters of 3,3,3-trifluoropyruvic acid have been shown to react easily with acrylamide (1) to form N-oxyamidoalkylation products (3a,b). As a result of the conversion data, methyl (3a) and ethyl (3b) esters of 2-acrylamino-3,3,3-trifluoro-2-hydroxypropionic acid are formed with a preparative yield of more than 90% (Scheme 3). It is also shown that in the initiation of AIBN 3a,b is polymerized [16].
Refluxing 3a in excess of thionyl chloride for 1,5-2 h results in quantitative replacement of OH-group with chlorine. The reaction is well-suited to TLC control in the ethylacetate-methylene chloride system = 1:3. Despite the aggressive reaction medium associated with the abundant release of HCl and SO2, no appreciable formation of by-products and polymerization products is observed.
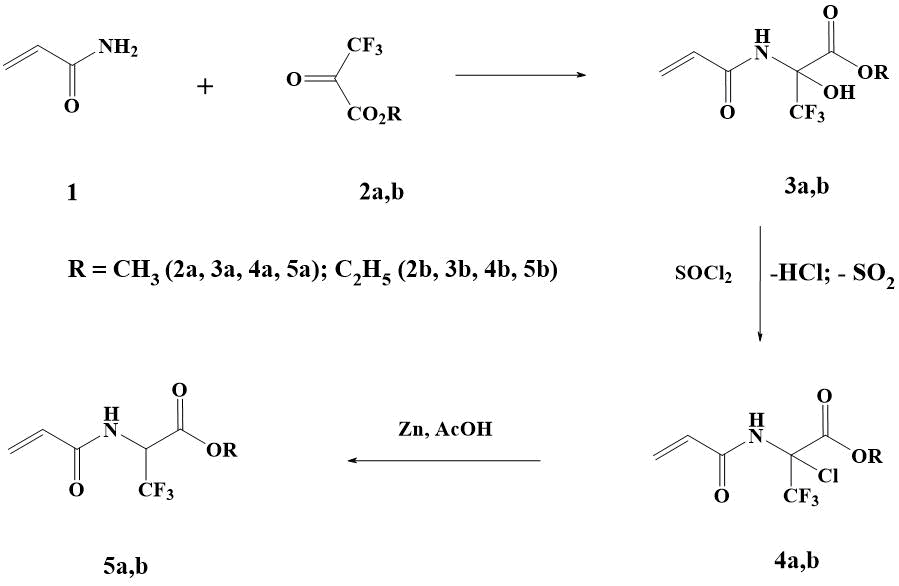
Scheme 3. Preparation of СF3- containing acrylamides.
After removal of excess SOCl2 and cooling, the reaction product spontaneously crystallizes. The spectrally pure 4a was obtained with 88% yield (Scheme 3). Ethyl ester of 2-acrylamino-2-chloro-3,3,3-trifluoropropionic acid 4b with yield 81% (Scheme 3) was obtained from amidooxy compound 3b by similar method.
It has previously been shown that the halogen, which is adjacent to the nitrogen atom of a carbon atom, in compounds of this structure is easily subjected to the reduction with triphenylphosphine with a yield of 57-85% [15]. In the present work we run on a cheaper method of recovery of chlorine derivatives 4a,b with zinc in acetic acid. The reduction of chlorine in compound 4a under these conditions has been found to occur exothermically and requires cooling of the reaction mass. After the standard isolation procedure (see Experimental part) and recrystallization, ester 5a was obtained in a yield of 61% (Scheme 3). Similarly, acrylate 5b was obtained from 4b with a yield of 62%.
The ability of the newly synthesized compounds 4a,b and 5a,b to react (co)polymerization is the subject of further study.
Experimental part
The 1H and 19F NMR spectra are recorded in D6-DMSO and CDCI3 using a Bruker Avance 300 device with operating frequencies (300 and 282 MHz, respectively). Chemical shifts in NMR spectra are given in the δ (ppm) scale relative to TMS as the internal standard (NMR spectra 1H) and CCl3F as an external standard (NMR spectra 19F). The spin-spin interaction constants are given in Hz. The reactions were monitored by TLC on Merck (silica gel 60 F254, 0.25 mm) plates. The RF synthesized compounds are defined in the ethyl acetate-methylene chloride system = 1:3. The elemental analysis is determined in the INEOS RAS laboratory of the microanalysis. Mass spectra are made on a quadrupole mass spectrometer FINNIGAN MAT INCOS 50, direct input, electron impact, ionization energy 70 eV.
Industrially available starting reagents - acrylamide [CAS 79-06-1], thionyl chloride [CAS 7719-09-7], as well as methyl [CAS 13089-11-7] and ethyl [CAS 13089-18-0] esters of 3,3,3‑trifluoropyruvic acid by SIA "P&M-Invest" Ltd are used.
2-Acrylamino-3,3,3-trifluoro-2-hydroxypropionic acid methyl ester (3a)
Obtained from acrylamide 1 and methyl 3,3,3-trifluoropyruvate 2a with output of 95% using the method described earlier [16]. M.p. 125-126°С (benzene).
2-Acrylamino-3,3,3-trifluoro-2-hydroxypropionic acid ethyl ester (3b)
Obtained from acrylamide 1 and ethyl 3,3,3-trifluoropyruvate 2b with output of 90% using the method described earlier [16]. M.p. 95-96°С (benzene).
2-Acrylamino-2-chloro-3,3,3-trifluoropropionic acid methyl ester (4a)
2.95 g (13 mmol) 3a and 6 ml of SOCl2 were placed in a glass pear-shaped flask equipped with a magnetic stirrer with heating and reflux. The reaction mass is refluxed for 2 hours with vigorous stirring (until the release of HCl and SO2). Excess SOCl2 and volatile reaction products were removed on a rotary evaporator. 3.1 g of viscous light yellow oil crystallizing in cooling are obtained. The crude product is dissolved in chloroform and passed through a layer of silica gel (D=20 mm, h=10 mm). The residues of the product are washed off the column with a small amount of chloroform and evaporated on a rotary evaporator to a constant weight. 2.8 g of oil crystallizing during cooling are obtained. Yield 88%, m. p. 85-86°C (CC14), Rf = 0.47.
1Н NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 7,08 (s, 1Н, NH), 6,22 (dd, 1Н, СН, 3JH-H(cis)=18, 3JH-H(trans)=12), 6,43 (br.d., 1H, Н2С=, 3JH-H=18), 5,86 (br.d, 1H, Н2С=, 3JH-H=12), 3,91 (с, 3 Н, ОСН3).
19F NMR spectrum (282 MHz, CDCl3, δ, ppm, J/Hz): -76,06 (с, 3F, CF3).
Found, %: С 34,14; Η 2,94; N 5.81. С7Н7ClF3NO3. Calculated, %: С 34,24; Η 2,87; N 5,70.
2-Acrylamino-2-chloro-3,3,3-trifluoropropionic acid ethyl ester (4b)
2.6 g (10 mmol) of 3b and 5 ml of SOCl2 are placed in a round-bottom glass flask equipped with a reflux condenser with a calcium chloride tube and a magnetic stirrer with heating. The reaction mass is refluxed for 2 hours with vigorous stirring until the release of SO2 is stopped. After removal of excess SOCl2 on the rotary evaporator, a viscous light-yellow oil is obtained, which crystallizes during cooling. The crude product is dissolved in chloroform and passed through a layer of silica gel (D=15 mm, h=10 mm). The residues of the product are washed off the column with a chloroform and evaporated on a rotary evaporator to a constant weight. The resulting residue is crystallized from cyclohexane. 2.1 g of white crystalline substance is obtained. Yield 81%, m. p. 75-76°C, Rf = 0.52.
1Н NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 6,94 (s, 1Н, NH), 6,44 (dd, 1Н, Н2С=, 3JH-H=18), 6,21 (br.d., 1H, СН, 3JH-H(cis)=18, 3JH-H(trans)=9), 5,85 (br.d, 1H, Н2С=, 3JH-H=9), 4,37 (q, 2Н, ОСН2, 3JH-H=6), 1,32 (t, 3Н, СН3, 3JH-H=6).
19F NMR spectrum (282 MHz, CDCl3, δ, ppm, J/Hz): -75,98 (с, 3F, CF3).
Found, %: С 36,94; Η 3,61; N 5.44; F 21,49. С8Н9ClF3NO3. Calculated, %: С 37,01; Η 3,49; N 5,40; F 21,95.
2-Acrylamino-3,3,3-trifluoropropionic acid methyl ester (5a)
245 mg (1 mmol) 4a and 1.1 ml of glacial acetic acid were placed in a round-bottom glass flask equipped with a reflux condenser, a calcium chloride tube and a magnetic stirrer. 600 mg of zinc in the form of dust is added to the reaction mass with vigorous stirring and at a temperature of 20°C. The reaction is carried out under these conditions for 1 hour. The reaction mass is then diluted with acetic acid and filtered. The filtrate was evaporated on a rotary evaporator to give a glassy residue. It was dissolved in 10 ml CH3CN, treated with 0.5 g SiO2 and the resulting slurry was passed through a thin layer of silica gel. After removing the solvent on a rotary evaporator, 200 mg of the desired product is obtained in the form of a white solid having trace amounts of the initial reagent 1 according to NMR 19F data. Recrystallization from aqueous methanol afforded analytically pure compound 5a as colorless elongated loose crystals. Yield 61%, m. p. 105-106°C, Rf = 0.59.
1Н NMR spectrum (300 MHz, D6-DMSO, δ, ppm, J/Hz): 9,16 (d, 1Н, NH, 3JH-H=9), 6,42 (dd, 1Н, СН, 3JH-H(cis)=18, 3JH-H(trans)=9), 6,24 (br.d., 1H, Н2C=, 3JH-H=18), 5,67 (br.d, 1H, Н2С=, 3JH-H=9), 5,36 (hept, 1Н, HС-CF3, 3JH-H=9, 3,82 (с, 3 Н, ОСН3).
19F NMR spectrum (282 MHz, D6-DMSO, δ, ppm, J/Hz): -71,15 (s, 3F, CF3).
Mass spectrum, m/z, (%): 211 (1) [M]+, 180 (1), 152 (8), 135 (5), 98 (9), 69 (1), 59 (4), 55 (100), 44 (1).
Found, %: С 39,71; Η 4,02; N 6.67; F 26,81. С7Н8F3NO3. Calculated, %: С 39,82; Η 3,82; N 6,63; F 26,99.
2-Acrylamino-3,3,3-trifluoropropionic acid ethyl ester (5b)
In a glass round-bottom flask equipped with a calcium chloride tube, a magnetic stirrer and a water bath, 260 mg (1mmol) 4b and 1.1 ml of glacial acetic acid were placed. With vigorous stirring and temperature of 20°C, 600 mg of zinc dust is added to the reaction mass. The reaction is carried out under these conditions for 30 minutes. The reaction mass is then diluted with 5 ml of acetic acid, stirred vigorously for 5-10 minutes at room temperature and filtered. The filtrate was evaporated on a rotary evaporator and a transparent dense residue was obtained. Its crystallization from aqueous methanol results in formation of 140 mg of spectrally and chromatographically pure compound 6 in the form of white fluffy crystals. Yield is 62%, m. p. is 102-103°C, Rf = 0.67.
1Н NMR spectrum (300 MHz, D6-DMSO, δ, ppm, J/Hz): 9,34 (d, 1Н, NH, 3JH-H=9), 6,40 (dd, 1Н, СН, 3JH-H(cis)=16, 3JH-H(trans)=9), 6,23 (br.d., 1H, Н2C=, 3JH-H=16), 5,77 (br.d, 1H, Н2С=, 3JH-H=9), 5,47 (hept, 1Н, HС-CF3, 3JH-H=9), 4,22 (q, 2 Н, ОСН2, 3JH-H=6), 1,33 (t, 3 Н, СН3, 3JH-H=6).
19F NMR spectrum (282 MHz, D6-DMSO, δ, ppm, J/Hz): -68,17 (s, 3F, CF3).
Mass spectrum, m/z, (%): 225 (1) [M]+, 180 (7), 153 (21), 84 (22), 69 (4), 55 (100), 50 (1), 43 (2), 28 (88).
Found, %: С 42.02; Η 4.77; N 6.29; F 24,53. С8Н10F3NO3. Calculated, %: С 42,67; Η 4,48; N 6,22; F 25,31.
Acknowledgements
The work was carried out within the framework of State Assignment No. 075-03-2023-642 of the Ministry of Science and Higher Education of the Russian Federation using the scientific equipment of the Center for Research on the Structure of Molecules of the INEOS RAS.
The authors express their gratitude to SIA "P&M-Invest" Ltd for the ethyl 3,3,3-trifluoropyruvate presented for research.
References
- M. Guerre, G. Lopez, В. Améduri, М. Semsarilar, V. Ladmiral, Polym. Chemistry, 2021, 12 (27), 3852–3877.
- K. Akamatsu, M. Shimada, T. Tsuruoka, H. Nawafune, S. Fujii and Y. Nakamura, Langmuir, 2010, 26, 1254–1259.
- K. Paek, H. Yang, J. Lee, J. Park and B. J. Kim, ACS Nano, 2014, 8, 2848–2856.
- R. J. Williams, A. M. Smith, R. Collins, N. Hodson, A. K. Das and R. V. Ulijn, Nat. Nanotechnol., 2009, 4, 19–24.
- Changkui Fu ∗, Ye Yu, Xin Xu, Qiaoyun Wang, Yixin Chang, Cheng Zhang, Jiacheng Zhao, Hui Peng, Andrew K. Whittaker, Progress in Polymer Science, 2020, 108, 101286.
- Jiayu Long, Jiankai Shan,Yaxin Zhao,Ying Ji,Hongwei Tan, and Huiliang Wang, Chem Asian J., 2021, 16, 2426 –2430.
- K. Kolouchova, O. Sedlacek, D. Jirak et al., Biomacromolecules, 2018, 19(8), 3515–3524.
- L. Lei, Qi Zhang, S. Shuxian, Z. Shiping, Polym. Chemistry, 2016, 7(34), 5456–5462.
- Patent US 2782184, 1953.
- Patent DE 1012920, 1957.
- Patent US 3324178, 1967.
- Patent 3549705, 1970.
- S. N. Osipov, A. F. Kolomiets, A. V. Fokin, Bulletin of the Academy of Sciences of the USSR, Division of Сhemical Sciences, 1988, 3, 122-126.
- Patent DE 3917835, 1990.
- A. Yu. Aksinenko, A. N. Pushin, V. А. Sokolov, Russian Chemical Bulletin, 2002, 51(11), 2136-2138.
- O. A. Melnik, A. A. Korlyukov, V. I. Dyachenko, Fluorine Notes, 2023, 3(148), 1-2.
ARTICLE INFO
Received 05 June 2024
Accepted 24 June 2024
Available online June 2024
Recommended for publication by
eLIBRARY Document Number (EDN) GJZCIA

Fluorine Notes, 2024, 154, 5-6
