Received: August 2024
DOI 10.17677/fn20714807.2024.04.01
Fluorine Notes, 2024, 155, 1-2
INVESTIGATION BY ROTATIONAL VISCOMETRY METHODS, NMR 119Sn SPECTROSCOPY AND QUANTUM CHEMISTRY OF THE EFFECT OF A CATALYTIC SYSTEM BASED ON TIN DI-n-BUTYLDILAURATE AND POLYFLUORINATED TETRAAMINE ON THE CURING PROCESS OF ELASTIC POLYURETHANES
S.V. Kudashev, I.A. Politsimako, V.F. Zheltobryukhov
Volgograd State Technical University
28 Lenin Avenue, Volgograd, 400005 Russia
e-mail: kudashev-sv@yandex.ru
Abstract: The modifying effect of the bisalkylation product tris-(2-aminoethylamine) 1H,1H,9H-trihydroperfluorononan-1-ol – polyfluorinated tetraamine on the curing process of polyurethane elastomers has been studied. Based on experimentally determined rate constants of the reaction mass viscosity, a synergistic catalytic effect of tin di-n-butyldilaurate and polyfluorinated tetraamine on the urethane formation reaction was revealed. Multicenter mechanisms of catalytic participation have been studied using NMR 119Sn spectroscopy and quantum chemical analysis.
Keywords: polyurethane elastomers, fluoropolymers, polyfluorinated amines, catalysis, organo-tin compounds, structure formation, modification, NMR spectroscopy, quantum chemical calculations, coordination bonds.
Introduction
Elastic polyurethanes are widely used as monolithic sports, roofing and waterproofing coatings [1-3]. The structure formation process of polyurethane elastomers is rather complicated and includes multiple chemical and physical-chemical processes combined to form a cross-linked polymer [3]. As catalysts of urethane-, biureo- and allophanate formation there can be used salts of metals (tin, cobalt, lead, zinc, copper, manganese, iron, cadmium, vanadium, bismuth, potassium, aluminum, cerium), tertiary amines and heterocyclic compounds (triethylamine, triethylenediamine, N,N-diethylcyclohexylamine, N,N,N/,N/-tetramethylbutane diamine, N-ethylmorpholine, pyridine), as well as their mixtures [4].
The unique structural feature of N-polyfluoroalkylation products of tris-(2-aminoethylamine) with polyfluorinated alcohols H(CF2CF2)nCH2OH is the varying substitution degrees presence of amino groups in the molecule and fluorinated fragment [5], which are collectively able to influence the physical and chemical assemblies ratio in the polyurethane mesh and the final material properties. Therefore, the study of curing polyurethane elastomers process in the presence of polyfluorinated tetraamines requires separate research.
The aim of the invention is to study the effect of a catalytic system based on tin di-n-butyldilaurate and a polyfluorinated amine synthesized by the bisalkylation of tris-(2- aminoethylamine) with a polyfluorinated alcohol (n=4), to a process for the curing of elastic polyurethanes by rotational viscosimetry, NMR 119Sn spectroscopy and quantum-chemical analysis (ab initio, DFT).
Experimental part
The elastomeric composition preparation.
Polymer compositions are obtained using a laboratory mixer by mixing (stirring speed 250 rpm-1) for 10 min 100 pts.wt oligoetherpolyol, 1 pts.wt chain branching agent, 1.5 pts.wt plasticiser, 1.5 pts.wt surfactant, 0.1 pts.wt urethane-formation catalyst and 1 pts.wt polyfluorinated tetraamine. Further, 100 pts.wt isocyanate was added to the reaction mass and stirred again for 7 minutes. The resulting mixture was poured into molds and kept at room temperature (cold curing method) until the Sh A hardness yield of the elastomer on the plateau.
Oligoetherpolyol Laprol 5003–2–B10 (TU 2226–023–10488057–95, PAO Nizhnekamskneftekhim) is a polymerization product of propylene oxide with glycerin, followed by block copolymerization with ethylene oxide with the following characteristics: hydroxyl number of 35 mg KOH/g, water mass fraction not more than 0.1%. The compositions curing was carried out with toluylene diisocyanate (the content of the 2,4-isomer was 80.5%) Desmodur T80 (Wanhua, China).
Grade grade glycerin (GOST 6259–75) was used as a chain branching agent. The urethane formation catalyst was tin di-n-butyldilaurate (in the form of a 2.5% solution in white spirit). DOA dioctyl adipate (GOST 8728-88) was used as the plasticizer. Oxyethylated monoalkylphenol Neonol AF 9-12 (TU 2483-077-05766801-98, PAO Nizhnekamskneftekhim) was used as the non-ionogenic surface-active substance.
Catalytic N-polyfluoroalkylation of tris-(2-aminoethylamine) 1H,1H,9H-trihydroperfluorononane-1-ol was carried out according to the procedure [5]. The bis-alkylation product (polyfluorinated tetraamine) is a yellow oil-like substance with boiling point 133-136°C (15 mm Hg. Ct).
Research Methods
Rheological properties were studied at 25±1°C (shear rate 1 s-1) on Brookfield DV-II+Pro and RPE polymer-1 with cylinder-cylinder working unit viscosimeters. The arithmetic mean of three parallel determinations is taken as a result of the test. Viscosity growth rate constants are calculated for two sections of re-kinetic curves anorphosis obtained by taking log on the axis of dynamic viscosity values η (Pa·s) for viscosity dependence on curing time (min) η–τ [6,7]. For all dependencies η–τ is typical the presence of different duration initial section (induction period), within which value η varies insignificantly, and then intensively increases according to law close to exponential. In coordinates lnη–τ, experimental dependencies have the form of two rectilinear sections with different angular coefficients and corresponding constants of the reaction mass Кη1 and Кη2 viscosity growth rate.
NMR spectra of 119Sn (149.22 MHz) in the form of the first derivative of the absorption signal are recorded at-5±0.2°C (to exclude chemical interaction between components) on a Bruker AVANCE 400 NMR spectrometer. Chemical shifts are indicated relative to Sn(CH3)4. The number of accumulations is not less than 6144 to obtain a good signal-to-noise ratio. Solutions in carbon tetrachloride with the following concentrations: toluylene diisocyanate – 0.05 mol/l, oligoetherpolyol – 0.10 mol/l, tin di-n-butyldilaurate – 0.03 mol/l, polyfluorinated tetraamine – 0.04 mol/l were prepared for spectral analysis.
The activation barriers of interactions, as well as the electron geometric structure of the molecules, were calculated in Gamess (ab initio, STO-3G**) and Priroda software products (DFT method using a non-empirical gradient approach and PBE functional in the TZ2P basis).
Results and discussion
The structural feature of the used modifier is the presence of a reactive primary amino group which, under conditions of migration polymerisation of diisocyanate and polyol, interacts with NCO groups of 2,4- and 2,6-toluylene diisocyanate to form disubstituted ureas (Scheme 1).
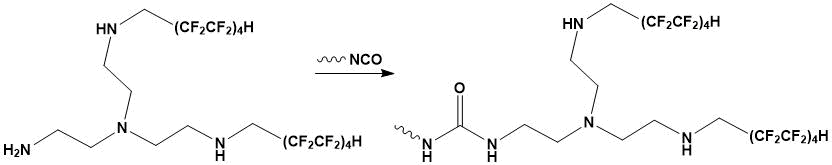
Scheme 1.
The study of the kinetics of curing of the polyurethane compositions made it possible to identify that the introduction of the modifier contributes to an increase in the reaction mass viscosity when measured under isothermal conditions (Table 1). The growth in values of the rate constants of the reaction mass viscosity increase in the case of injection of the polyfluorinated tetraamine is due to the main catalysis of the urethane formation process involving a tertiary nitrogen atom.
Table 1. Values of reaction mass viscosity increase rate constants during polyurethane elastomers formation.
|
Catalytic system |
Rate constant Кη (25 °С) |
|
|
Кη1·10-2, min-1 |
Кη2·10-2, min-1 |
|
|
tin di-n-butyldilaurate |
0,80 |
3,10 |
|
polyfluorinated tetramine |
0.43 |
1,95 |
|
tin di-n-butyldilaurate – polyfluorinated tetramine |
1,80 |
5,30 |
Possible mechanisms of catalytic participation of tin di-n-butyldilaurate and polyfluorinated tetramine (separately, together) in the reaction of diisocyanate and polyol leading to urethane formation have been investigated by methods of quantum-chemical analysis and molecular modeling. The analysis is carried out under conditions in which the reaction coordinate was fixed according to the O-H bond of the oligoetherpolyol. According to the data of the DFT density functional method, the formation of associates, as well as the coordination bond C=O→SnIV and O→SnIV in the “tin-organic catalyst – toluylene diisocyanate – polyol” system, contributes to an energy gain of 310.5 kJ/mol. For a “polyfluorinated amine – toluylene diisocyanate – polyol” system (taking into account the interactions of N→SnIV, HN→SnIV and H2N→SnIV), this value is significantly lower and is 157.2 kJ/mol, which is consistent with the data on the determination of the rate constants Kη, according to which the polyfluorinated tetramine occurs on the catalytic activity of tin di-n-butyldilaurate.
The use of a catalyst system based on an organotin catalyst and a polyfluorinated tetramine provides maximum energy gain of 394.7 kJ/mol. Donor-acceptor complexes formation due to vacancy 5d-orbitals of SnIV tin di-n-butyldilaurate and non-divided oxygen and nitrogen electron pairs of isocyanate groups and polyfluorinated tetraamine collectively will contribute to the most effective elongation (“activation”) of polyol (denoted as R-OH) OH-bond and facilitating the urethane formation (Scheme 2).
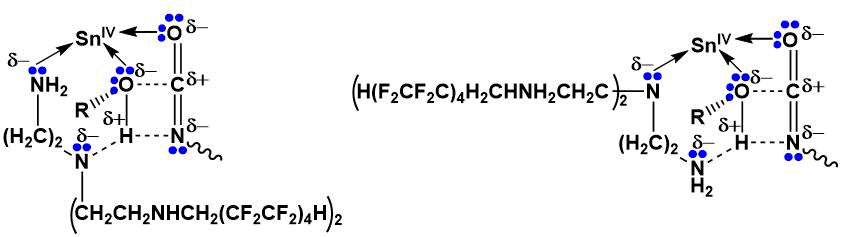
Scheme 2.
According to the results of calculations performed by the ab initio method, a change in the dipole moment of the interacting particles with 5.18 D (in the absence of a polyfluorinated tetraamine) to 7.60 D is observed for a system including simultaneously tin di-n-butyldilaurate and a polyfluorinated tetraamine, as well as a decrease in the charge on the diisocyanate NCO group nitrogen atom from –0.269 to –0.377. Positive charge on oxygen atom of polyol OH-group increases from 0.233 to 0.279. Furthermore, the H-O bond elongation occurs (the bond length H-O of the oligoetherpolyol increases from 0.956 Å in the presence of an organotin catalyst to 2.218 Å using a bicomponent catalyst system). Such increase of distance between atoms indicates communication break and proton transition to nitrogen atom with urethane group formation. The energy barrier of the urethane formation reaction with the participation of tin di-n-butyldilaurate is 208.5 kJ/mol, and with the additional introduction of the polyfluorinated tetraamine the energy barrier is already 153.1 kJ/mol.
The effect on the position, width and shape of the tin di-n-butyldilaurate resonant lines spectrum with the addition of the polyurethane formulation (Figure 1) corresponding components studied by NMR 119Sn experimental spectroscopy methods. The spectrum of the initial tin di-n-butyldilaurate is represented by a wide line with an extremum at H ~ 8512 G and a chemical shift in the region δ1 = –590,2 – 665,2 ppm, and a narrow central line with δ2 = –653,5 ppm. Decomposition of this resonance line into components shows that these lines are close to a Gaussian shape, but have different amplitudes (the relative intensity of the narrow line is 1.3 times greater than the intensity of the wide line). The resonant line asymmetric broadening is due to intramolecular polynuclear interactions (С=O→SnIV, O→SnIV), and the narrow line corresponds primarily to the contribution of intermolecular polynuclear interactions (“peripheral” effect).
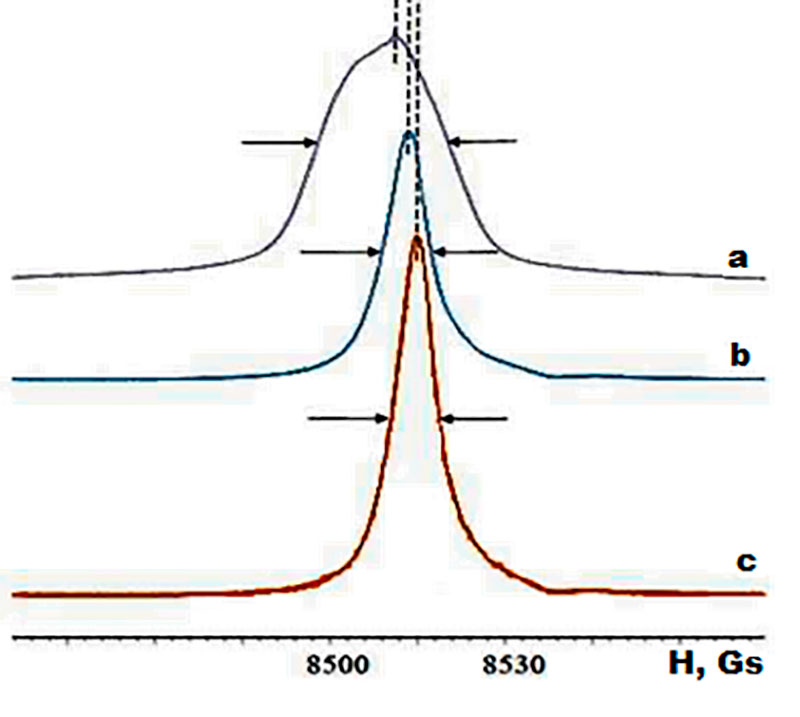
Figure 1. Experimental spectra of NMR 119Sn: a – tin di-n-butyldilaurate;
b – tin di-n-butyldilaurate + toluylene diisocyanate + oligoether polyol;
c – tin di-n-butyldilaurate + polyfluorinated tetraamine + toluylene diisocyanate + oligoether polyol. H – magnetic field.
Introduction of toluylene diisocyanate, oligoetherpolyol and, finally, polyfluorinated tetraamine results in form, width and position transformation of observed line. The only one dominant narrow resonant line with a tendency for its narrowing and preservation of asymmetry in the NMR spectrum is observed along with the shift of the maximum. Such changes are related to the reorganization of the coordination environment SnIV by involving different structural fragments (amino groups with the different degree of tetraamine substitution and NCO groups with a linear structure) into multi-center donor-acceptor interactions resulting in a single signal with a maximum relative intensity. The presence of a weakly expressed arm in the area H ~ 8539 G corresponds to the contribution of products formed during partial absorption of water vapor by toluene diisocyanate to form another type of coordination with SnIV.
Thus, the effect of a catalytic system based on tin di-n-butyldilaurate and a polyfluorinated tetraamine on the curing elastic polyurethanes process has been investigated and shows that there is a synergistic effect of accelerating the urethane formation reaction associated with the formation of multi-site polyassociative interactions arising between an organotin catalyst, tetraamine, toluylene diisocyanate and oligoetherpolyol, which collectively contribute to facilitating the formation of urethane.
References
- Thomas S., Datta J., Haponiuk J. et al, Polyurethane Polymers: Composites and Nanocomposites, Elsevier, Amsterdam, Netherlands, 2017, 634 p.
- Galimberti M., Rubber-Clay Nanocomposites. Science, Technology, and Applications, John Wiley & Sons Limited, 2011, 627 p.
- Clemitson I. R., Castable Polyurethane Elastomers, CRC Press (Taylor & Francis Group), 2015, 272 p.
- Entelis S. G., Nesterov O. V., Kinetika i mekhanizm reakcij izocianatov s soedineniyami, soderzhashchimi «aktivnyj» vodorod, Uspekhi Khimii, 1966, 35 (12), 2178-2203. (in Russian)
- Kudashev S. V., Sidelnikov V. S., Politsimako I. A., Zheltobryukhov V. F. Synthesis and NMR study of the N-polyfluoroalkylation product of tris(2-aminoethyl)amine with 1H,1H,9H-trihydroperfluoronan-1-ol, Fluorine notes, 2024, 2 (153), 3-4.
- Nistratov, A. V., Fiziko-himicheskie principy razrabotki receptur i tekhnologii kompozicij na osnove oligotiolov, oligodienov i oligoefirov, ispol'zuemyh dlya polucheniya polimernyh materialov s uluchshennymi tekhniko-ekspluatacionnymi harakteristikami: Dis. ... doktora tekhn. nauk: 02.00.06, Volgograd, 2014, 448 с. (in Russian)
- Kudashev S. V., Modifikaciya ryada geterocepnyh polimerov kompoziciyami na osnove poliftorirovannyh spirtov i montmorillonita: Dis. ... doktora khimicheskih nauk: 02.00.06, Volgograd, 2020, 283 с. (in Russian)
ARTICLE INFO
Received 09 August 2024
Accepted 23 August 2024
Available online August 2024
Recommended for publication by PhD O. V. Bryzgalova
eLIBRARY Document Number (EDN) WDKOQV

Fluorine Notes, 2024, 155, 1-2
