Received: August 2024
DOI 10.17677/fn20714807.2024.04.02
Fluorine Notes, 2024, 155, 3-4
MICROCALORIMETRIC STUDY OF THE INTERACTION OF TOLUENE DIISOCYANATES WITH POLYFLUORINATED TETRAAMINES
S.V. Kudashev1, A.V. Faleva2, I.A. Politsimako 1, V.F. Zheltobryukhov1
1 Volgograd State Technical University
28 Lenin Avenue, Volgograd, 400005 Russia
e-mail: kudashev-sv@yandex.ru
2 Core Facility Center «Arktika»,
M.V. Lomonosov Northern (Arctic) Federal University
14 Severodvinskaya str., Arkhangelsk, 163002 Russia
Abstract: The reactions of polyfluorinated tetraamines synthesized by the interaction of tris-(2-aminoethylamine) with polyfluorinated alcohols, with 2,4- and 2,6-toluenediisocyanates under conditions of catalysis with tin di-n-butyldylaurate were studied by isothermal microcalorimetry. The values of the enthalpy change in the reaction of diisocyanate with amino groups of varying degrees of substitution have been determined. The influence of the chemical structure of polyfluorinated tetraamine on the change in the enthalpy of the reaction has been established. The possible contribution of a three-substituted nitrogen atom to the mechanism of catalysis of reacting molecules in the process of chain elongation and branching is shown.
Keywords: substituted urea, fluoropolymers, polyfluorinated amines, catalysis, organotin compounds, microcalorimetry, change in enatalpy.
Introduction
The reactions of isocyanates with active hydrogen-containing compounds are used to produce not only individual substances, but also oligomers and polymers [1–3]. Thus, the catalytic reaction of isocyanates with polyols results in polyurethane elastomers which have been used as coatings for various purposes [4]. Operation characteristics of these coatings (simultaneous exposure to UV radiation, aggressive environment, photochemical and microbiological destruction, surface abrasion) lead to multiple destruction processes of the cross-linked polymer.
The special significance to stabilize the elastic polyurethanes properties are reactive poly-and perfluorinated compounds which can be introduced both at the polymer synthesis stage (migration polymerisation of di-, polyisocyanate and polyol) and by surface modification of finished products [5–7]. Due to the multicomponent nature of real formulations used to produce monolithic sports, roofing and waterproofing coatings [4], the synthesis of novel fluorine-containing modifiers for polyurethane elastomers is of interest, containing several reaction centers.
Products of tris-(2-aminoethylamine) N-polyfluoroalkylation by polyfluorinated alcohols H(CF2CF2)nCH2OH [8] can be assigned to the number of such modifying additives. Synthesized polyfluorinated tetramines contain amino-groups of different degree of substitution and fluorinated fragment in their structure. The combination of the centers different in reactivity can have a complex effect on the curing process, the structure and properties of the final material. In this regard, the study of the reaction features of aromatic isocyanates with polyfluorinated tetraamines is interesting to improve the formulations of polyurethane elastomers.
The aim of the invention is a microcalorimetric study of isomeric 2,4- and 2,6-toluenediisocyanates and polyfluorinated amines reaction, synthesized by bis-alkylation of tris-(2-aminoethylamine) with polyfluorinated alcohols (n = 3, 4) under conditions of catalysis with tin di-n-butyldilaurate.
Experimental part
Microcalorimetric studies in isothermal mode were carried out on Calve C80 Setaram microcalorimeter (resolution of 0.1 mcW) at 25°C. The reagents (first section: 1 pts.wt tetramine + 0.1 pts.wt organotin catalyst; second section: 100 pts.wt diisocyanate) were poured into a membrane calorimetric cell which prevents mixing thereof during thermostating. The thermostated substances were mixed by puncturing the membrane with a special device. The comparison cell was empty. Nominal cell volume was 12.5 ml, diameter is 17 mm. The reaction mass was stirred by means of a freely falling jet when the calorimetric cell membrane was punctured. Error rate in determining heat amount in experiment did not exceed 3%. The arithmetic mean of three parallel measurements was taken as the final result of the mixing enthalpy. The preliminary stage of calorimetric experiments has studied the effect of membrane puncture effects and mixing of reagents, the type of adhesion cell, as well as the presence or absence of misuse interleaving on the reproducibility of experimental data, and the conditions for carrying out the calamus of the metric analysis [9] were determined, which ensure good convergence of the results obtained. Preliminary stage of calorimetric experiments has studied effect of membrane puncture effects and mixing of reagents, type of comparison cell, as well as presence or absence of mixing on experimental data reproducibility and calorimetric analysis [9] conditions were determined, providing good convergence of obtained results. At the same time, it has been appreciated that elastic polyurethanes formulations components within a wide range of temperatures (up to boiling) are limited compatible substances, have a distinct interface between the phases on interferograms and the absence of the components interdiffusion [4].
The electron geometric structure of the molecules was calculated in the Gamess (ab initio, STO-3G**) software product.
Toluene diisocyanate (the content of the 2,4-isomer was 80.5%) of the grade Desmodur T80 (Wanhua, China) was used as a diisocyanate. Tin di-n-butyldilaurate (Kosmos 19, China) was used in the form of a 2.5% solution in white spirit.
Method of synthesis of polyfluorinated tetraamine
1 ml (6.67 mmol) of Tris(2-aminoethyl)amine and 2.53 ml (13.34 mmol) 1H,1H,7H- trihydroperfluoroheptane-1-ol in the presence of catalytic amounts of montmorillonite (0.1 mg) was dispersed at ultrasound frequency of 40 kHz in a sealed glass ampoule at 80°C for 2 hours, followed by heating to 120°C for 6 hours. Initial reagents were separated from the reaction product by extraction of cooled i-PrOH at –6°С…0°С with further fractional vacuum distillation of the extract. Yield is 2.17 g (42%), yellow oil, Rf was 0.68, t of boiling was 129-132°C (15 mm Hg. Ct).
NMR spectrum 1Н [CDCl3], δ, ppm: 1,50 s (2Н, H2N), 1,52–1,98 m (12Н, СН2СН2), 2,98 t (4Н, СH2CF2, J = 24 Hz), 5,29 t.t (2Н, HCF2, J1 = 12 Hz, J2 = 54 Hz), 7,27 s (2H, HNCH2).
NMR spectrum 13С [CDCl3], δ, ppm: 36,46–39,44 (CH2NH2), 43,87 (CH2CF2), 47,09 (CH2NHCH2CF2), 50,19–57,20 (NCH2CH2NH), 58,85 (NCH2CH2NH2), 106,04–115,95 (CF2).
NMR spectrum 19F [CDCl3], δ, ppm: –121,83…–122,40 м (CF2), –123,70…–129,76 (CF2CН2), ‑140,40 (CF2Н).
Found, %: С 31,00; Н 2,84; N 7,21. C20H22F24N4. Calculated, %: С 31,02; Н 2,86; N 7,24. М 774,38.
Reaction of tris(2-aminoethyl)amine and polyfluorinated alcohol (1:2 mol.) occurs according to the equation (Scheme 1).
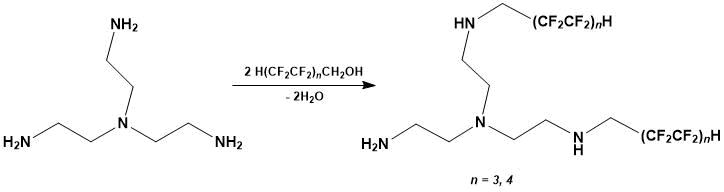
Scheme 1.
To compare the effect of the polyfluorinated tetraamine chemical structure by the amount of change in the enthalpy of reaction with toluene diisocyanates, calorimetric studies have included tetraamine experiments (n = 4), which was synthesized by reacting tris (2 - aminoethyl) amine and 1H,1H,9H- trihydroperfluorononane-1-ol. Synthesis and identification of said compound are described in [8].
According to gas-liquid chromatography and mass spectroscopy data (for spectra, the presence of a molecular ion peak [М]+ с Irel = 12–40 %) in the reaction products of tris(2-aminoethyl)amine with said polyfluorinated alcohols at a molar ratio of 1:2 is detected not only the product of bis-alkylation, but also mono-, trialkylation (table 1).
Table 1. Ratio between tris (2-aminoethyl)-amine N-polyfluoroalkylation products with polyfluorinated alcohols
|
Polyfluorinated alcohol |
Content,% vol. |
||
|
Monoalkylation product |
Bisalkylation product |
Trialkylation product |
|
|
H(CF2CF2)3CH2OH |
3,1 |
96 |
0,9 |
|
H(CF2CF2)4CH2OH |
4,5 |
95 |
0,5 |
Tris(2-aminoethyl)amine (95%, Keyingchem, China) was purified by distillation and had the following characteristics: boiling point 114°C (15 mm Hg. Ct), d 0.976 g·ml-1, n20D 1.497. Polyfluorinated alcohol 1H,1H,7H-trihydroperfluoroheptane-1-ol (≥ 95%, HaloPolymer, Perm) has t. boiling 170°C (760 mm Hg. Ct) and d 1.75 g·ml-1. Calcined montmorillonite clay (≥ 98%, JSV B-Clay, Kazakhstan) has specific surface area of 595 m2·g-1 (by water) and 64 m2·g-1 (by nitrogen), cation exchange capacity of 100 mg-eq/100 g and is presented in a mixture form of three main fractions: 50–100 nm – 10 wt.%, less than 1 mcm – 80 wt.%, less than 10 mcm – 10 wt.%.
NMR spectra were recorded at 25°C on a Bruker AVANCE III (600 MHz) device in СDCl3 (1H – 600,30 MHz, 13С – 161,99 MHz, 19F – 376 MHz), using Si(CH3)4 as the internal standard. The chemical shifts of nuclei 19F are defined relative to CFCl3. Standard pulse sequences, including two-dimensional HSQC and HMBC experiments, were used to record all spectra. Topspin 3.2. software package was used for all spectra processing.
Quantitative chromatograph mass-spectral analysis was carried out on a Shimadzu GCMS-QP2010 SE device: a capillary quartz column HP-5 MS with a length of 30 m, a carrier gas – helium. Programmable column heating from 80 to 280°C, evaporator temperature – 250°C. Elemental analysis was carried out on a CHNS/O Euro EA3100 analyzer. TLC was carried out on Sorbfil plates (Russia), eluent – chloroform-acetone (1:1).
Results and discussion
The interaction of isocyanates with primary and secondary amine groups of the polyfluorinated tetraamine leads to the substituted ureas formation (Scheme 2). The amino groups reactivity in this case is determined not only by their basicity but also by steric factors [10]. According to the given quantum-chemical calculation by the ab initio method (for polyfluorinated tetraamine n = 3), the charges on nitrogen atoms are: NH2-groups q = - 0.283; NH-groups q = - 0.250. Further chain elongation and branching is possible as a result of the interaction of isocyanate groups with NH groups of the urea moiety, which are characterized by low reactivity. These reactions are possible in the process of polyurethane and polyurethane-urea elastomers post-curing [4, 6].
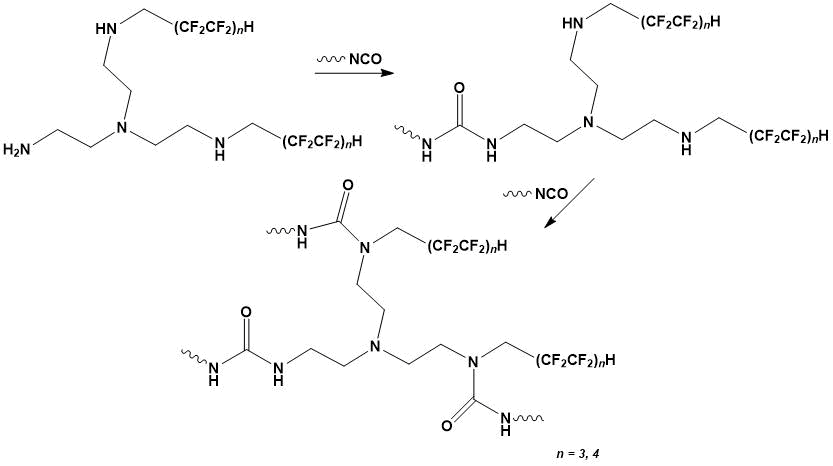
Scheme 2.
The results of the calorimetric studies are presented in Table 2. For the dependences of heat flow (mW) on time (min) W=f(τ), two well-allowed exothermic peaks are typical (peak intensity of the first exothermic effect 10 times greater than the intensity of the second exo-effect). Both peaks correspond to a change in the enthalpy of reaction of 2,4- and 2,6-toluene diisocyanates, preferably with primary (indicated by index 1) and secondary (index 2) amino groups. It is necessary to consider that the NCO-groups in the para-position of the benzene ring are more reactive than in the ortho-position.
Analysis of the obtained data indicates the dependence of the change amount in the reaction enthalpy from the polyfluorinated tetraamine chemical structure. The accumulation of fluorine in the tetraamine molecule creates not only steric hindrance (taking into account possible intramolecular non-covalent interactions), but also leads to a decrease in the tetraamine basicity, and therefore a partial increase in the time to reach ΔH.
Table 2. The effect of the polyfluorinated tetraamine chemical structure on the reaction enthalpy change (ΔH) with toluene diisocyanates and the time (τ) of reaching the ΔH value.
|
Tetraamine H2N–CH2CH2–N(CH2CH2–HN–CH2(CF2CF2)nH)2 |
–ΔН1, kJ/mol |
τ1, min |
–ΔН2, kJ/mol |
τ2, min |
|
n = 3 |
62,8 |
15 |
14,5 |
18 |
|
n = 4 |
49,4 |
24 |
10,0 |
29 |
The specific catalytic effect of tin compounds on the reaction of isocyanates with active hydrogen-containing compounds is related to the possibility of donor-acceptor complexes formation due to vacancy 5d-orbitals of tin [10]. However, the tertiary nitrogen accumulation in the chain branching process can also contribute to the ΔH2 amount (Scheme 3).
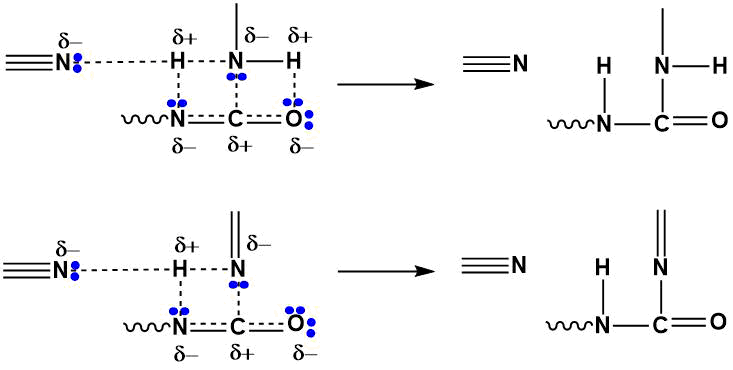
Scheme 3.
In this case, the H-N bond activation (elongation) in the primary and secondary amino groups results from the polarization with a tertiary nitrogen atom not only of the starting polyfluorinated tetraamine, but the catalytic effect of the reaction products containing N≡. The degree of polarization is directly related to the basicity of the amine and adjacent structural fragments shielding effect. According to quantum-chemical calculations, the polarization of the H-N bond (the length of the bond increases, on average, from 1.02 Å to 2.02 Å) with the participation of tertiary nitrogen provides an increase in the dipole moment of the interacting particles (tetraamine n=3) from 5.09 D to 6.12 D (with the participation of tetraamine n = 4 – up to 6.00 D).
Thus, microcalorimetric examination in isothermal mode of reaction of polyfluorinated tetraamines with a mixture of isomeric toluene diisocyanates is characterized by the presence of two exothermic effects of corresponding step-by-step interaction of isocyanate groups of diisocyanate with primary and secondary amine groups of tetraamines to form substituted ureas.
Acknowledgements
NMR spectra were recorded using Core Facility Center «Arktika» of the Northern (Arctic) Federal University» instrumentation.
References
- Thomas S., Datta J., Haponiuk J. et al., Polyurethane Polymers: Composites and Nanocomposites, Elsevier. Amsterdam, Netherlands, 2017, 634 p.
- Galimberti M., Rubber-Clay Nanocomposites. Science, Technology, and Applications, John Wiley & Sons Limited, 2011, 627 p.
- Clemitson I. R., Castable Polyurethane Elastomers, CRC Press (Taylor & Francis Group), 2015, 272 p.
- Nistratov, A. V., Fiziko-himicheskie principy razrabotki receptur i tekhnologii kompozicij na osnove oligotiolov, oligodienov i oligoefirov, ispol'zuemyh dlya polucheniya polimernyh materialov s uluchshennymi tekhniko-ekspluatacionnymi harakteristikami: Dis. ... doktora tekhn. nauk: 02.00.06, Volgograd, 2014, 448 р. (in Russian).
- Kudashev S. V., Methods of introducing poly- and perfluorinated fragments in to a macromolecular system (Review), Fluorine notes, 2020, 3 (130), 3-4.
- Kudashev S. V., Modifikaciya ryada geterocepnyh polimerov kompoziciyami na osnove poliftorirovannyh spirtov i montmorillonita: Dis. ... doktora khimicheskih nauk: 02.00.06, Volgograd, 2020, 283 р. (in Russian).
- Kudashev S. V., Terekhov A. A., Babkin V. A., Nistratov A. V., Zheltobryuhov V. F., Andreev D. S., Ignatov A. V., Arisova V. N., Bogdanov A. I., Kuznecova N. V., Iznos kompozita na osnove poliuretana, poliftorirovannogo spirta, montmorillonita i politetraftoretilena, Trenie i iznos, 2023, 44 (2), 135-141. (in Russian).
- . Kudashev S. V., Sidelnikov V. S., Politsimako I. A., Zheltobryukhov V. F., Synthesis and NMR study of the N-polyfluoroalkylation product of tris(2-aminoethyl)amine with 1H,1H,9H-trihydroperfluoronan-1-ol, Fluorine notes, 2024, 2 (153), 3-4.
- Kal've E., Prat A., Mikrokalorimetriya. Primenenie v fizicheskoj himii i biologii, Moskva, Khimiya, 1963, 478 p. (in Russian).
- Entelis S. G., Nesterov O. V., Kinetika i mekhanizm reakcij izocianatov s soedineniyami, soderzhashchimi «aktivnyj» vodorod, Uspekhi Khimii, 1966, 35 (12), 2178-2203. (in Russian).
eLIBRARY Document Number (EDN) KPTVVS
ARTICLE INFO
Received 16 August 2024
Accepted 27 August 2024
Available online August 2024
Recommended for publication by PhD M.A. Manaenkova
eLIBRARY Document Number (EDN) KPTVVS

Fluorine Notes, 2024, 155, 3-4
