Received: October, 2015
DOI 10.17677/fn20714807.2015.06.02
Fluorine Notes, 2015, 103, 5-6
Synthesis of 3-perfluoroalkylpropanals and 3-perfluoroalkylpropionitriles
Anikó Nemes, Máté Berta, Peter Ivanko, Dénes Szabó and József Rábai*
Institute of Chemistry, Eötvös Loránd University, P. O. Box 32, H-1518, Budapest 112, Hungary
* E-mail: rabai@elte.hu
Abstract: Simple method for the preparation of the title fluorous propionaldehydes using silver ion assisted dehydroiodination of 3-perfluoroalkyl-2-iodo-1-propanols (F-iodohydrins) and their effective conversion to the corresponding fluorous nitriles with N,O-bis(trifluoroacetyl)-hydroxylamine is described.
Keywords: Fluorous building blocks, F-iodohydrins, dehydroiodination, fluorous Pomeroy reaction.
Introduction of some Rfn-groups into organic molecules is a method to synthesize fluorous compounds. [1]. The fluorous phase affinity of these molecules mostly controlled by the length of the fluorous chain (n in Rfn = CnF2n+1); however the reactivity of these compounds depend on the number of methylene spacers between the highly electron withdrawing perfluoroalkyl substituent and the functional group. Changing these variables allows the fine tuning of both phase properties and reactivities of such fluorous compounds. [2]Fluorous aldehydes are important building blocks and have been used for the synthesis of recoverable 2° and 3° fluorous amines, [3] and of the fluorous derivatives of racemic and enantiomerically pure 1-phenylethylamine. [4] Recently, fluorous aldehydes were used as analytical reagents for the determination of the concentration of 1° biogen amines in human plasma samples via their double reductive N,N-fluoroalkylation reaction to form an easy to separate 3° fluorous amine derivative {QN[(CH2)3Rf8]2} [5]. Fluorinated nitriles are useful raw material for lubricants, fiber-, leather- or paper processing additives, corrosion inhibitors and surfactants [6].
Literature methods for the preparation of the title aldehydes involve either sequential functional group transformations of F-carboxylic acids (QFCO2H → QFCOCl → QFCH2OH → QFCH=O) [7], or fluorous ethyliodides (Rfn(CH2)2I → Rfn(CH2)2Li → Rfn(CH2)2CHO) [8]; or oxidation of fluorous alcohols (QFCH2OH → QFCH=O) [3,9] or the use of convergent synthetic strategies such as hydroformylation reactions of perfluoroalkyl-ethenes (RfnCH=CH2) using Co2(CO)8 [10], or Rh/phosphine [11] catalyst.
Preparation of fluorous nitriles based on convergent synthesis strategies, using the reaction of industrial raw materials such as perfluoroalkyl idodides [12], -bromide [12 a-d] or -chloride [13], (RfnX, X = I, Br, Cl) with acrylonitrile; perfluoroalkyl-ethenes (RfnCH=CH2) with HCN or cyanohydrines [6]; and perfluoroalkyl ethyl iodides (RfnCH2CH2I) with alkali cyanides [14].
In the present work we describe the synthesis of perfluoroalkylpropanals { Rfn(CH2)mCH=O [Rfn = CnF2n+1, n = 4, 6, 8, 10; m =2]; (F-aldehydes)} and perfluoroalkylpropionitriles { Rfn(CH2)mCN [Rfn = CnF2n+1, n = 4, 6, 8, 10; m =2]; (F-nitriles)} using 3-perfluoroalkyl-2-iodo-1-propanols (F-iodohydrins; 2a-d) as starting materials. These presursors can be obtained easily by the radical addition reaction of perfluoroalkyl iodides 1a-d to allylic alcohols [15] and serve as a platform [16] for the synthesis of F-propanols (RfnCH2CH2CH2OH) [17], F-propenols (RfnCH=CHCH2OH) [18],and F-propenes (RfnCH2CH=CH2) [19] as well. Following a literature precedent [20] we choose halohydrin to aldehyde transformation as a means for synthesizing valuable fluorous aldehydes 3a-d.
Our novel procedure involves the silver nitrate assisted dehydroiodination reaction of iodohydrins 2a-d in a mixture of tert-butanol and water at 60 °C temperature to afford the target aldehydes 3a-d in acceptable yields (Scheme 1). However, due to the sensitive nature of these aldehydes - which are prone to form trimers [(RfnCH2CH2CHO)3] [21] on the action of acids, on longer standing, or being heated - great care should be applied during their isolation, purification and storage (see Experimental part).
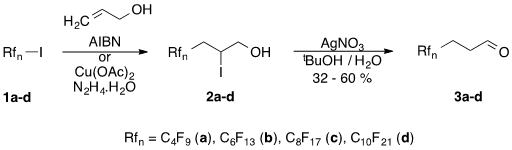
Scheme 1. Two step synthesis of F-aldehydes starting from perfluoroalkyl iodides.
We also studied the reaction of these fluorous aldehydes with N,O-bis(trifluoroacetyl)-hydroxyl amine, which is a reagent that allows the transformation of organic aldehydes to the corresponding nitriles under mild reaction conditions, as first published by Pomeroy and Craig [22]. We found that this reaction could be used for the synthesis of 3-perfluoralkylpropionitriles with good to excellent yields (fluorous Pomeroy reaction) (Scheme 2).
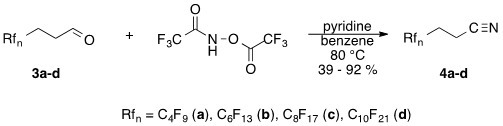
Scheme 2. Synthesis of F-propionitriles from their precursor F-aldehydes.
Although the above fluorous variant of the Pomeroy reaction is a powerful tool for small scale synthesis of fluorinated nitriles using safe reagents and very mild reaction conditions, on large scale it could not compete with literature methods suitable for production of F-propionitriles.
Experimental
F-iodohydrins (2a-d) [17,23] and N,O-bis(trifluoroacetyl)-hydroxylamine [22] were prepared as reported. 1H, 13C and 19F NMR spectra were recorded on Brucker Avance 250 instrument using a 5 mm 1H/13C/31P/19F quad probe head at room temperature (298 K). Chemical shifts (δ) are given in parts per million (ppm) units relatively to the internal standard 1% TMS (δ = 0.00 for 1H, δ = 0.00 for 13C) and to 0.5% CFCl3 as internal standard (δ = 0.00 for 19F). Melting points were determined on a Boetius micro-melting point apparatus and are not corrected. The reactions were monitored and the product analyzed by gas chromatography (Hewlett-Packard 5890 Series II, PONA [crosslinked methylsilicone gum] 50 m × 0.2 mm × 0.5 μm column, H2 carrier gas, FID detection; Program: 120 °C, 5 min, 10 °C/min, 250 °C, 5 min; Injector temperature: 250 °C, Detector temperature: 280 °C). The IR spectra were recorded on Bruker Alpha fourier transformation IR spectrometer, solid sample in KBr pastille, liquid sample film between KBr windows.
General procedure for synthesis of 3-perfluoroalkylpropanals (3a-d)
To the mixture of F-iodohydrine 2a-d (10 mmol) in tert-butanol (10 ml) the solution of AgNO3 (2.04 g, 12 mmol) in water (5 ml) was added. The reaction mixture was heated to 60 °C (internal temperature, oil temperature: 75 °C) and was stirred for 3 hours. Then the reaction mixture was cooled to room temperature, filtered and the solid material was washed with ether (10 ml). The phases of the two-phase filtrate was separated, the organic phase was washed with water (3 × 10 ml) and dried over Na2SO4. The solvent was removed under vacuo, and the crude product was purified by column chromatography (10 × 3 cm silicagel, eluent: pentane-dichloromethane = 1:1) or by vacuum distillation.
4,4,5,5,6,6,7,7,7-Nonafluoroheptanal (3a)
The mixture of 2a (5.00 g; 12.4 mmol) in tBuOH (10 ml) and AgNO3 (2.52 g; 15 mmol) in water (5 ml) were reacted to yield 1.1 g (32 %) colorless oil with GC purity of 99.1 %; showing agreable NMR data to that reported in [11b]. 1H NMR (CDCl3) δ: 2.47 (m, 2H, CF2CH2); 2.83 (t, 2H, 3JHH = 7.5 Hz, CH2CHO), 9.84 (s, 1H, CHO). 19F NMR (CDCl3) δ: -81.57 (m, 3F, CF3); -115.03 (m, 2F); -125.03 (m, 2F); -126.59 (m, 2F).
4,4,5,5,6,6,7,7,8,8,9,9,9-Tridecafluorononanal (3b)
The mixture of 2b (5.00 g; 10.0 mmol) in tBuOH (10 ml) and AgNO3 (2.02 g; 12 mmol) in water (5 ml) were reacted to yield 1.77 g (48 %) colorless oil with GC purity of 94.8 %. 1H NMR (CDCl3) δ: 2.46 (m, 2H, CF2CH2); 2.83 (t, 2H,3JHH = 7.5 Hz, CH2CHO), 9.84 (s, 1H, CHO). 13C NMR (CDCl3, partial) δ: 24.0 (t, 3JCF = 22.0 Hz, CF2CH2); 35.1 (CH2CHO); 198.3 (CHO).19F NMR (CDCl3) δ: -81.42 (tt, 3F, 3JFF = 10.0 Hz, 5JFF = 2.2 Hz, CF3); -114.94 (m, 2F); -122.47 (m, 2F); -123.49 (m, 2F); -124.10 (m, 2F); -126.77 (m, 2F).
4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-Heptadecafluoroundecanal (3c)
The mixture of 2c (10.0 g; 16.6 mmol) in tBuOH (20 ml) and AgNO3 (3.10 g; 18.2 mmol) in water (10 ml) were reacted to yield 3.8 g (48 %) white waxy solid, with GC purity of 95.9 %; showing agreable NMR data to that reported in [11b]. 1H NMR (CDCl3) δ: 2.47 (m, 2H, CF2CH2); 2.82 (t, 2H, 3JHH = 7.5 Hz, CH2CHO), 9.83 (s, 1H, CHO). 13C NMR (CDCl3, partial) δ: 24.0 (t, 3JCF = 23.4 Hz, CF2CH2); 35.1 (CH2CHO); 198.3 (CHO). 19F NMR (CDCl3) δ: -81.48 (t, 3F, 3JFF = 9.9 Hz, CF3); -115.00 (m, 2F); -122.71 (m, 6F); -123.49 (m, 2F); -124.18 (m, 2F); -126.93 (m, 2F).
4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,13-Heneicosafluorotridecanal (3d)
The mixture of 2d (10.00 g; 14.2 mmol) in tBuOH (20 ml) and AgNO3 (2.89 g; 17.0 mmol) in water (10 ml) were reacted to yield 3.5 g (43 %) white waxy solid, with GC purity of 91.6 %. 1H NMR (CDCl3) δ: 2.47 (m, 2H, CF2CH2); 2.83 (t, 2H, 3JHH = 7.5 Hz, CH2CHO), 9.84 (s, 1H, CHO). 13C NMR (CDCl3, partial) δ: 24.2 (t, 3JCF = 24.1 Hz, CF2CH2); 34.9 (CH2CHO); 198.2 (CHO). 19F NMR (CDCl3) δ: -81.51 (t, 3F, 3JFF = 10.0 Hz, CF3); -112.52 (m, 2F); -115.00 (m, 2F); -122.71 (m, 8F); -123.49 (m, 2F); -124.18 (m, 2F); -126.93 (m, 2F).
Upscaled procedure for the synthesis of 3-perfluorooctylpropanal (3c)
To the mixture of 4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoro-2-iodopropan-1-ol (2c, 300 g, 0.50 mol) in tert-butanol (600 ml) the solution of AgNO3 (93 g, 0.55 mol) in water (300 ml) was added. The reaction mixture was heated to 60 °C (internal temperature, oil temperature: 75 °C) and was stirred for 3 hours. Then the reaction mixture was cooled to room temperature, filtered and the solid material was washed with ether (300 ml). The phases of the two-phase filtrate was separated, the organic phase was washed with water (3 × 300 ml) and dried over Na2SO4. The solvent was removed under vacuo, and the crude product was purified by vacuum distillation (bp = 78-84 °C/1 mmHg). Yield: 141.2 g (60 %). Note: Bath temperature should not exceed 100 °C and the whole preparation must be executed on the same day, since overnight standing or higher bath temperature results in the drop of yield and purity.
General procedure for the synthesis of 3-perfluoroalkylpropionitriles (4a-d)
The solution of 3-perfluoroalkylpropanal 3a-d (2.5 mmol), N,O-bis(trifluoroacetyl)-hydroxylamine (0.56 g; 2.5 mmol) and pyridine (0.40 g; 5.0 mmol) in benzene (4 ml) was heated for 1 h at 80 °C. The solvent was removed under vacuo, the solid residue was dissolved in ether (10 ml) and washed with 1M HCl (5 ml) and water (3 × 10 ml). After drying over Na2SO4 the solvent was removed in vacuo. The crude product was purified by column chromatography (8 × 1.5 cm silicagel, eluent: pentane-dichloromethane = 1:1) or by recrystallization from 2,2,4-trimethylpentane (isooctane).
4,4,5,5,6,6,7,7,7-Nonafluoroheptanenitrile (4a)
The mixture of 3a (0.9 g; 3.3 mmol) N,O-bis(trifluoroacetyl)-hydroxylamine (0.73 g; 3.3 mmol) and pyridine (0.52 g; 6.5 mmol) in benzene (4 ml) was reacted to yield 0.35 g (39 %) colorless oil with GC purity of 90.1 %. (Lit. bp = 64 °C/5 mmHg [24].) 1H NMR (CDCl3) δ: 2.52 (m, 2H, CH2CN); 2.70 (m, 2H, CF2CH2).19F NMR (CDCl3) δ: -81.58 (tt, 3F, CF3,3JFF = 9.5 Hz, 5JFF = 3.0 Hz); -116.13 (m, 2F); -124.95 (m, 2F); -126.58 (m, 2F). IR (film): 3145, 1665, 1401, 1359, 1230, 1135, 1052, 1015, 981, 881, 881, 853, 749, 735, 713, 599.
4,4,5,5,6,6,7,7,8,8,9,9,9-Tridecafluorononanenitrile (4b)
The mixture of 3b (1.0 g; 2.7 mmol), N,O-bis(trifluoroacetyl)-hydroxylamine (0.60 g; 2.7 mmol) and pyridine (0.42 g; 5.4 mmol) in benzene (4 ml) was reacted to yield 0.90 g (91 %) colorless oil, with GC purity of 90.9 %.(Lit. bp = 79 °C/5 mmHg [25].) 1H NMR (CDCl3) δ: 2.51 (m,2H, CH2CN); 2.69 (m, 2H, CF2CH2). 19F NMR (CDCl3) δ: -81.45 (tt, 3F, CF3,3JFF = 9.8 Hz, 5JFF = 2.7 Hz); -115.96 (m, 2F); -122.46 (m, 2F); -123.46 (m, 2F); -124.05 (m, 2F); -126.77 (m, 2F). IR (film): 2983, 2258, 1691, 1446, 1390, 1367, 1319, 1240, 1146, 1122, 1075, 1020, 981, 926, 846, 812, 780, 746, 732, 709, 697, 653, 568, 533.
4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-Heptadecafluoroundecanenitrile (4c)
The mixture of 3c (1.0 g; 2.1 mmol), N,O-bis(trifluoroacetyl)-hydroxylamine (0.47 g; 2.1 mmol) and pyridine (0.33 g; 4.2 mmol) in benzene (4 ml) was reacted to yield 0.91 g (92 %) white solid of mp = 70-72 °C with GC purity of 95.6 %. (Lit. mp = 64 - 66 °C [26] and Lit. bp = 106 °C/5 mmHg [24].) 1H NMR (CDCl3) δ: 2.52 (m, 2H, CH2CN); 2.70 (m, 2H, CF2CH2). 19F NMR (CDCl3) δ: -81.18 (t, 3F, CF3,3JFF = 10.0 Hz); -115.77 (m, 2F); -122.11 (m, 2F); -122.33 (m, 4F); -123.15 (m, 2F); -123.82 (m, 2F); -126.56 (m, 2F). IR (KBr): 2257, 1374, 1335, 1203, 1147, 11115, 1078, 1037, 980, 921, 873, 705, 662, 606, 560, 531.
4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,13-Heneicosafluorotridecanenitrile (4d)
The mixture of 3d (1.0 g; 1.74 mmol), N,O-bis(trifluoroacetyl)-hydroxylamine (0.39 g; 1.74 mmol) and pyridine (0.28 g; 3.5 mmol) in benzene (4 ml) was reacted to yield 0.91 g (92 %) white solid of mp = 102-104 °C, with GC purity of 95.6 %. 1H NMR (CDCl3) δ: 2.52 (m, 2H, CH2CN); 2.70 (m, 2H, CF2CH2). 19F-NMR (CDCl3) δ: -81.19 (t, 3F, CF3,3JFF = 9.7 Hz); -115.77 (m, 2F); -122.18 (m, 10F); -123.14 (m, 2F); -123.81 (m, 2F); -126.56 (m, 2F). IR (KBr): 3424, 2258, 1641, 1445, 1376, 1345, 1211, 1151, 1112, 1080, 982, 884, 751,712, 665,647, 556, 530.
References
- (a) Kiss, L. E.; Kövesdi, I.; Rábai, J. An Improved Design of Fluorophilic Molecules: Prediction of the ln P Fluorous Partition Coefficient, Fluorophilicity, Using 3D QSAR Descriptors and Neural Networks, J. Fluorine Chem., 2001, 108, 95-109; (b) Rábai, J.; Szlávik, Z.; Horváth, I. T. Chemistry in Fluorous Biphasic Systems, Chapter 22, in: Handbook of Green Chemistry and Technology, Eds. Clark, J.; Maquarrie, D. Blackwell, 2002, Oxford, pp. 502-523.
- (a) Gladysz, J. A.; Curran, D. P.; Horváth, I. T. (Eds.) Handbook of Fluorous Chemistry Wiley-VCH, Weinheim, 2004; (b) Fluorous Chemistry in Topics in Current Chemistry, Ed. Horváth, I. T.; Springer, Heidelberg, 2012; (c) Lo, A. S. W.; Horvath, I. T. Fluorous ethers (Critical Review), Green Chem., 2015, 171, 4701-4714.
- Rocaboy, C.; Bauer, W.; Gladysz, J. A. Convenient Syntheses of a Family of Easily Recoverable Fluorous Primary, Secondary, and Tertiary Aliphatic Amines NH3-x[(CH2)m(CF2)7CF3]x (m = 3-5; x = 1-3) – Fine Tuning of Basicities and Fluorous Phase Affinities, Eur. J. Org.Chem., 2000, 14, 2621-2628.
- Szabó, D.; Nemes, A.; Kövesdi, I.; Farkas, V.; Hollósi, M.; Rábai, J. Synthesis and characterization of fluorous (S)- and (R)-1-phenylethylamines that effect heat facilitated resolution of (±)-2-(8-carboxy-1-naphthylsulfinyl)benzoic acid via diastereomeric salt formation and study acid via diastereomeric salt formation and study, J. Fluorine Chem., 2006, 127, 1405-1414.
- Hayama, T.; Sakaguchi, Y.; Yoshida, H.; Itoyama, M.; Todoroki, K.; Yamaguchi, M.; Nohta, H. Binary Fluorous Alkylation of Biogenic Primary Amines with Perfluorinated Aldehyde Followed by Fluorous Liquid Chromatography−Tandem Mass Spectrometry Analysis, Anal. Chem., 2012, 84, 8407-8414.
- (a) Oishi, T. Preparation of polyfluoronitriles, Jpn. Kokai Tokkyo Koho (1992), JP 04149167 A 19920522; (b) Oishi, T. Preparation of polyfluoronitrile by hydrogen cyanide addition to polyfluoro-α-olefin, Jpn. Kokai Tokkyo Koho (1992), JP 04154752 A 19920527; (c) Oishi, T. Preparation of fluorine-containing nitriles by addition reaction of hydrogen cyanide to fluorine-containing olefins, Jpn. Kokai Tokkyo Koho (1992), JP 04198157 A 19920717; (d) Ooishi, T. Preparation of fluorine-containing nitriles, Jpn. Kokai Tokkyo Koho (1993), JP 05331127 A 19931214.
- Dawson, G. W.; Mudd, A.; Pickett, J. A.; Pile, M. M.; Wadhams, L. J. Convenient synthesis of mosquito oviposition pheromone and a highlyfluorinated analog retaining biological activity, J. Chemical Ecology, 1990, 16(6), 1779-89.
- Chu, Q.; O’Neal, K.; Osipov, M.; Ngwendson, J. N.; Geib, S. J.; Weber S. G.; Curran D. P. Synthesis, characterization, and applications of fluorous resorcin[4]arenes, New J. Chem., 2010, 34, 2732-2734 and Supporting Information.
- Lévêque, L.; Le Blanc, M.; Pastor, R. Synthesis of per(poly)fluoroalkyl aldehydes RF(CH2)nCHO, Tetrahedron Lett., 1998, 39 (48), 8857-8860.
- 10 Hoechst AG, Frankfurt. Improvements in and relating to the preparations of fluorinated aldehydes and alcohols, GB 1414323 (1975).
- (a) Wrackmeyer, B.; Werner, K. Von; Wehowsky, F. 17O And13 C Nuclear Magnetic Resonance Study of Polyfluorinated Carbonal Compounds, J.Fluorine Chem., 1987, 35, 359-372; (b) Ohtsuka, Y.; Kobayashi, O.; Yamakawa T. Highly selective hydroformylation of 3,3,3-trifluoropropene to 4,4,4-trifluorobutanal using Rh/Xantphos catalyst, J. Fluorine Chem., 2014, 161, 34-40.; (c) Yamakawa, A.; Otsuka, Y.; Kobayashi,O. Process for the preparation of 3-(perfluoroalkyl)propanals, Jpn Kokai Tokkyo Koho (2015) JP 2015086200 A (20150507).
- (a) Hu, C.; Qiu, Y. Cobaloxime-catalyzed hydroperfluoroalkylation of electron-deficient alkenes with perfluoroalkyl halides: reaction and mechanism, J. Org. Chem. 1992, 57(12), 3339-42. DOI:10.1021/jo00038a022; (b) Barata-Vallejo, Sebastian; Postigo, Al; (Me3Si)3SiH-Mediated Intermolecular Radical Perfluoroalkylation Reactions of Olefins in Water, J. Org. Chem., 2010, 75(18), 6141-6148; (c) Commeyras, A.; Blancou, H.; Moreau, P.; Functionalizing perfluorinated residues, Ger. Offen. (1977), DE 2708751 A1 19770908; (d) Blancou, H. J.; Commeyras, A. A. A.; Teissedre, R. Compounds having a perfluoroalkyl group prepared in acid media containing zinc, Eur. Pat. Appl. (1981), EP 38735 A1 (19811028); (e) Hu, C.; Qiu, Y. Cobaloxime-catalyzed hydroperfluoroalkylation of electron-deficient alkenes with perfluoroalkyl halides: reaction and mechanism, J. Org. Chem., 1992, 57(12), 3339-42.
- Huang, X.-T.; Chen, Q.-Y. Nickel(0)-Catalyzed Fluoroalkylation of Alkenes, Alkynes, and Aromatics with Perfluoroalkyl Chlorides, J. Org. Chem., 2001, 66(13), 4651-4656.
- Foulletier, L.; Lalu, J. P. Polyfluorinated nitriles, Fr. (1969), FR 1560544 19690321.
- Igumnov, S. M.; Don, V. L.; Vyazkov, V. A.; Narinyan, K. E. Copper salt-catalysed reaction of perfluoroalkyl halides with olefins, Mendeleev Commun., 2006, 16 (3), 189-190.
- Ivanko, P.; Takács, F. T.; Rábai, J. Convergent synthesis of fluorous reagents, 14th European Symposium on Fluorine Chemistry, Poznań, Poland, 2004. Lecture. Book of Abstracts, p. 84.
- Rábai, J.; Szíjjártó, Cs.; Ivanko, P.; Szabó, D. 3-(Perfluoroalkyl)propanols: Valuable Building Blocks for Fluorous Chemistry, Synthesis, 2007, 2581-2584.
- Ivanko, P.; Szíjjártó, Szabó, D.; Rábai, J. Method for the synthesis of pure (E)-3-(perfluoroalkyl)-allylic alcohols, Fluorine notes, Vol. 5 (84), 2012. /public/2012/5_2012/letters/rusletter2.html; /public/2012/5_2012/letters/letter2.html
- 19 Szíjjártó, Cs.; Ivanko, P.; Takács, F. T.; Szabó, D.; Rábai, J. Syntheses of fluorous propenes from 3-perfluoroalkyl-2-iodo-1-propanols, J. Fluorine Chem,. 2008, 129, 386-389.
- (a) Bougault, J. Action of iodine and mercuric oxide on styrene and safrole, Compt. Rend., 1900, 131, 528-30. (b) Schulze, K.; Habermann, A.-K. ; Uhlig, H.; WyRuwa, K.; Himmelreich, U. Synthesis, Structure and Reactions of Epoxyfencholene Aldehyde, J. Prakt. Chem., 1993, 335, 363-367; (c) Schulze, K.; Uhlig, H. Verfahren zur Herstellung von isomerem Campholenaldehyd, DD 254382 A1 (24.02.1988).
- Laurent, P.; Blancou, H.; Commeyras, A. Synthèse de Fluoroalkyl éthanal: RFCH2CHO, Tetrahedron Lett., 1992, 33 (18), 2489-2492.
- Pomeroy, J. H.; Craig, C. A.Two New Synthesis of Nitriles from Aldehydes, Using O,N-Bis-(Trifluoroacetyl)-Hydroxylamine or Trifluoracetohydroxamic Acid, J. Am. Chem. Soc., 1959, 81, 6340-6341.
- ЗАО НПО <<ПиМ-Инвест>> Синтезы фторорганических соединений, Под редакцией Игумнова С.М.; Игумновой Э.В., Москва 2011, Часть 2, Глава 1, c. 20-29, 31-32.
- New polyfluorinated nitriles and their production (Ugine Kuhlmann, a French Body Corporate). GB 1244256 (1971).
- Blancou, H. J.; Commeyras, A. A. A. Procédé de préparation de produits ayant un groupement perfluoroalkyle en présence de zinc en milieu acide, EP0038735 (1981).
- Bříza, T.; Kvíčala, J.; Paleta, O.; Čermák, J. Preparation of bis(polyfluoroalkyl)cyclopentadienes, new highly fluorophilic ligands for fluorous biphase catalysis, Tetrahedron, 2002, 58, 3841-3846.
Recommended for publication by V. Kornilov
Fluorine Notes, 2015, 103, 5-6
